Engineering, Environment
Corrosion inhibition and adsorption characteristics of Tridax Procumbens L leaves extract on mild steel immersed in 1M HCl solution
Kenneth Kanayo ALANEME*, Adetomilola Victoria FAJEMISIN, Sunday Joseph OLUSEGUN, Joseph Olatunde BORODE
Department of Metallurgical and Materials Engineering, Federal University of Technology, Akure, P.M.B 704, Nigeria
*Corresponding author, E-mail: kalanemek@yahoo.co.uk; +2348034228868
Abstract
The corrosion inhibition and adsorption mechanism of Tridax procumbens Linn leaves extract on mild steel immersed in 1M HCl solution was investigated. Mass loss, corrosion rate and inhibition efficiency from gravimetric and potentiodynamic polarization measurements, atomic adsorption spectroscopy (AAS), Fourier transform infra red spectroscopy (FTIR), and scanning electron microscopy; were used for the analysis. The results show that within the selected range of concentration of the extract and corrosion test temperatures, the inhibition efficiency obtained was within the range of 81 and 96 %. The efficiency values were observed to increase with increase in the extract concentration but decreased slightly with increase in temperature. The inhibitor molecules were noted to be physically adsorbed on the mild steel surface with OH, NH, and CO groups confirmed as the active phytochemical constituents in the extract responsible for the adsorption. The adsorption process fitted perfectly with the Langmuir adsorption isotherm indicating that the extract was strongly adsorbed on the mild steel surface.
Keywords
Mild steel; Corrosion; Fourier transform infra red spectroscopy (FTIR); Inhibition efficiency; Potentiodynamic polarization analysis; Tridax procumbens Linn leaves extract
Introduction
The mitigation of corrosion and its adverse effects on materials in service have continued to receive the attention of researchers. In the industrial sector, there are notable processes such as crude oil refining, petrochemical processing, industrial cleaning, acid pickling and descaling among others, where ferrous materials are used as primary component parts/machineries [1]. In most of the highlighted processes, there are frequent contacts between the steel parts and acidic solutions resulting in varied scales of corrosion. Traditional mitigation strategies include the use of coating, cathodic protection and electroplating among others. The paradigm shift towards green technology has helped to sustain the growing interest in the use of agricultural plants and their derivatives as sources for the development of inhibitors [2-3]. Their advantages include low cost of processing, availability and biodegradability [3]. Also, some of the plants used as green inhibitors are weeds with very little economic or established medicinal values. From open literature, it is appreciated that the corrosion inhibition characteristics of several plants have been explored, some of which are: Avogado Nuts[2]; Camellia Sinensis[3]; TithoniaDiversifolia[4]; Eugenia jambolana[5]; Eclipta Alba[6]; Hunteria Umbellata [7]; Atropa Belladonna[8]; Ficus Abutilifolia[9]; Pavetta Indica[10]. Tridax procumbemns L. which is a very common herb [11-12] has surprisingly received very little attention judging from the very little literatures available on its corrosion inhibition potentials.
Our interest in studying the corrosion inhibition potentials of Tridax procumbemns L is motivated by its chemical constitution. The plant is noted to contain chemical constituents such as alkaloids, flavonoids, carotenoids, β-sitisterol, n-hexane, fumaric acid, luteolin, quercitin, oxoester, lauric acid, myristic, palmitic, arachidic, linoleic acid and tannin; which are common constituents in plants with known corrosion inhibiting properties [13-14].
The present study evaluates the corrosion inhibition and adsorption characteristics of Tridaxprocumbens L. leaves extract on mild steel immersed in 1 M HCl solution.
Material and method
Materials
Mild steel of composition Fe=98.3%, C=0.133%, P=0.0061%, Mn=0.82%, Cr=0.08% was selected as the metallic substrate for this study. The steel was mechanically cut to coupons of dimension 20 x 10 cm, and then polished using different grades of emery paper. Analar grade HCl and distilled water were used for the preparation of the acid solution while Tridax procumbens Linn (TPL) leaves was used as a source of corrosion inhibitor.
Inhibitor preparation
Tridax procumbens Linn (TPL) leaves were obtained within the environs of the Federal University of Technology, Akure, Nigeria. The leaves of the plant were gathered, sun dried and pulverized. About 20g of the pulverized leaves was weighed and added to 200ml of prepared 1M HCL solution in a reagent bottle. The mixture was immersed in a water bath maintained at 90oC for 3 hours, after which it was removed from the water bath and allowed to cool overnight. The mixture was then filtered to obtain the extract. Stock solutions of 0.1, 0.2, 0.3, 0.4, and 0.5% (v/v) TPL leaves extract in 1M HCl were prepared from the filtrate.
Gravimetric analysis
Weight loss studies were carried out on pre-weighed mild steel samples which were immersed into 100ml of the blank/inhibitor solutions maintained at 303,313, 323 and 333K in a thermostat controlled water bath. The substrates were held in the solutions for 4 hours after which they were reweighed and the difference in weight taken as weight loss. From the weight loss data collated, the corrosion rates (CR), inhibition efficiency (IE) and surface coverage (θ) were calculated using equations (1)-(3), respectively in accordance with [1]:
CR = ΔW/(A∙T) (1)
where CR (in mg∙h-1∙cm-2) is corrosion rate, ∆W is weight loss (in mg), A is the area of the coupon (in cm2) and T is time (in hours).
When CRinh and CRblank correspond to the corrosion rates in the presence and absence of inhibitor respectively, then IE is the inhibition efficiency (in percents):
IE = 100∙(1-CRinh/CRblank) (2)
The surface coverage (θ) is calculated using:
θ = 1-CRinh/CRblank (3)
Mass loss measurement
Pre-weighed mild steel samples were immersed in 100ml of the blank (without inhibitor)/inhibitor solutions for 7 days (168 hours). The weight loss of each coupon was determined at every 24 hour intervals by retrieving the samples from the solution, cleaning and then reweighing. The mass loss for each sample was evaluated by dividing its weight loss by the surface area of the test sample [4].
Potentiodynamic polarization measurements
The electrochemical experiments were carried out using AUTOLAB PGSTAT 204N instrument, piloted by Nova software. The experiments were performed using a three-electrode corrosion cell set-up comprising of the mild steel as the working electrode, saturated silver/silver chloride as reference electrode, and platinum rod as counter electrode. The test set-up and testing procedure was in accordance with ASTM G59-97 [15] standard. The working electrode was immersed in a test solution (1M HCl) for 30 minutes until a stable open circuit potential was attained. The working electrodes were prepared by attaching an insulated copper wire to one face of the sample using an aluminum conducting tape, and cold mounting it with epoxy resin. Potentiodynamic polarization measurements were carried out using a scan rate of 1.0 mV/s at a potential initiated at -250mV to + 250mV with respect to OCP. After each experiment, the electrolyte and the test sample was replaced. The linear Tafel segments of the anodic and cathodic curves were extrapolated to corrosion potential to obtain the corrosion current densities (Icorr) and corrosion potential (Ecorr).
Atomic adsorption spectroscopy (AAS) analysis
Atomic adsorption analysis was performed using atomic adsorption spectrometer model bulk 200. This was required to determine the concentration of iron (II) ions in the 1 M HCl solutions after gravimetric measurements. The calibration curve of iron (II) ions was drawn before analyzing the electrolyte solution. All samples containing iron ions were diluted with distilled water to ensure that the concentrations of metal ions are within the range of the calibration curve.
Infrared measurements
The chemical functional groups present in the extract were determined using IR spectroscopic analysis [16]. Finely powdered (iron filing) mild steel was immersed in a solution of hydrochloric acid containing TPL extract for 4 hours to form the adsorption product of mild steel and extract. FTIR spectrum was recorded for the extracts and the adsorption product. The spectra were recorded in a Perkin-Elmer-1600 spectrophotometer using KBr pellet.
Scanning electron microscopy analysis
The surface morphology of the corroded substrates with and without the addition of the extract was investigated with the aid of scanning electron microscopic (SEM) analysis. Secondary electron imaging from a JSM 7600FJeol ultra-high resolution field emission gun scanning electron microscope (FEG-SEM) was utilized for the studies.
Results
Table 1. Corrosion parameters from Potentiodynamic polarisation curves for mild steel in 1M HCl solution in the absence and presence of different concentrations of TPL leaves extract
|
Conc (%) |
Ecorr (mV) |
Icorr (µA/cm2) |
Corr Rate (mmpy) |
|
Blank |
-449.008 |
480.132 |
5.5753 |
|
0.1 |
-445.447 |
103.82 |
1.2056 |
|
0.2 |
-439.709 |
95.739 |
1.1117 |
|
0.3 |
-445.45 |
93.426 |
1.0849 |
|
0.4 |
-443.699 |
66.593 |
0.7733 |
|
0.5 |
-444.62 |
56.436 |
0.65534 |
Table 2. Values of thermodynamic parameters: activation energy, enthalpy and entropy of activation
|
Acid medium |
Concentration of acid extract of Gliricidiasepium (% v/v) |
Activation energy, Ea (kJ∙mol-1) |
Enthalpy of activation, ∆Ha (kJ∙mol-1) |
Entropy of activation, ∆Sa (J∙mol-1∙k-1) |
|
1 M HCl |
Blank |
47.82 |
45.18 |
-78.67 |
|
1 |
54.00 |
51.36 |
-74.07 |
|
|
2 |
55.59 |
52.95 |
-71.16 |
|
|
3 |
57.59 |
54.95 |
-66.95 |
|
|
4 |
57.99 |
55.35 |
-67.30 |
|
|
5 |
69.58 |
66.94 |
-34.53 |
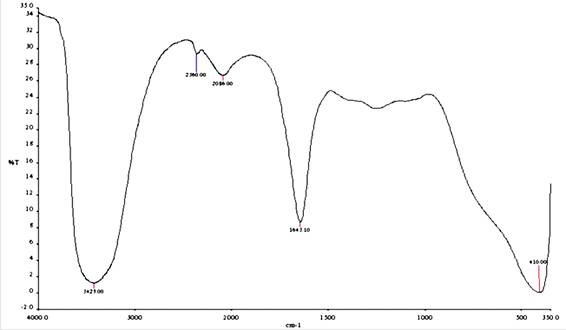
Figure 1. FT-IR spectrum of acid extract of TPL leaves
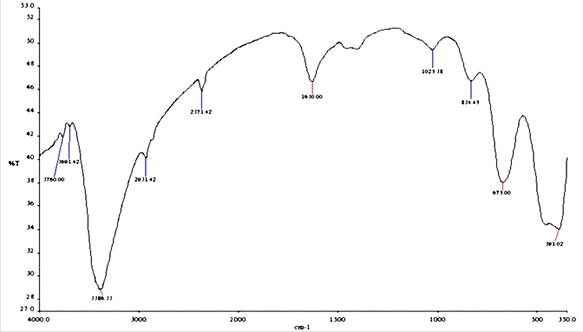
Figure 2. FT-IR spectrum of corrosion product of mild steel in the presence of acid extract of TPL in 1M Hydrochloric acid
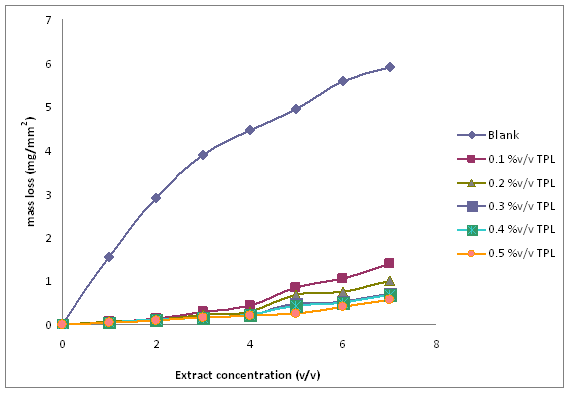
Figure 3. Plot of mass loss (mg/mm2) against exposure time (days) for the corrosion of mild steel in1M HCl, in the absence and presence of different concentration of TPL leaves extract
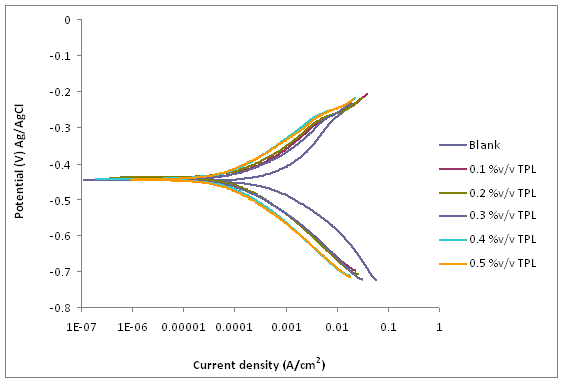
Figure 4. Potentiodynamic polarisation curves for mild steel in 1M HCl solution in the absence and presence of different concentrations of TPL leaves extract
|
|
(a) |
|
|
(b) |
|
|
(c) |
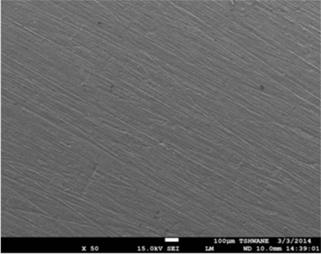
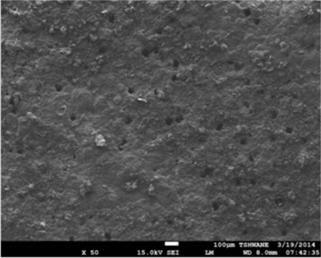
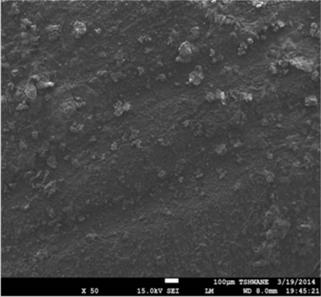
Figure 5. SEM secondary electron images showing surface morphology of the mild steel substrates, (a) before immersion in the 1M HCl solution, (b) after immersion test in the 1M HCl solution without the extract, and (c) after immersion test in the 1M HCl solution containing 0.3v/v % TPL extract.
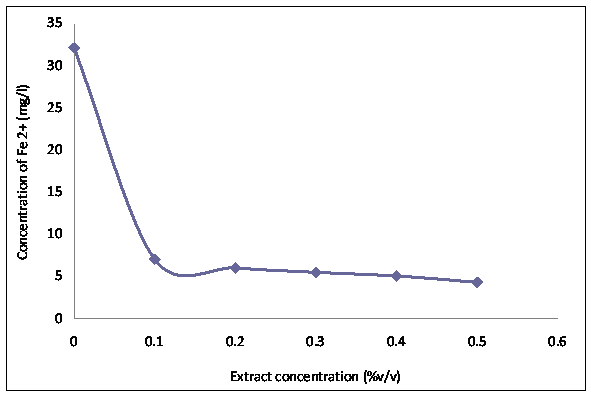
Figure 6. Graphs of the concentrations of iron (II) ions in 1M hydrochloric acid after weight loss measurements
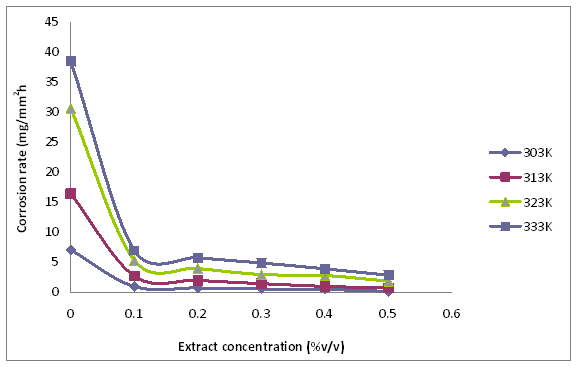
Figure 7. Effect of concentration and temperature on corrosion rate of mild steel in the presence and absence of Tridax procumbens L leaves extract
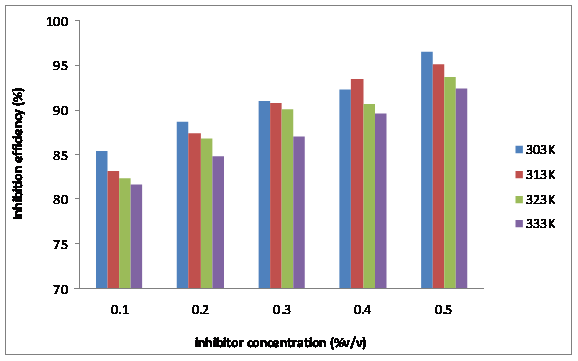
Figure 8. Effect of concentration and temperature on the corrosion of mild steel in the presence of different concentration of TPL leaves extract
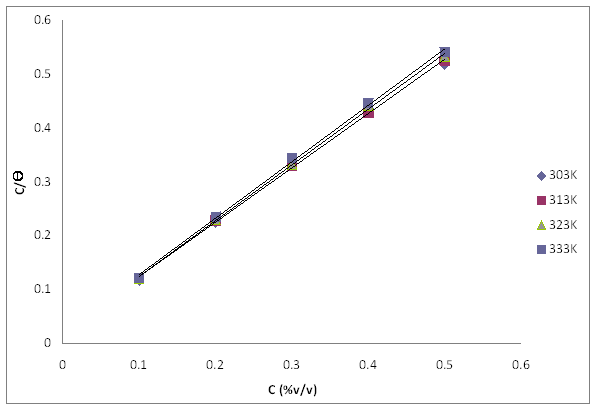
Figure 9. Langmuir adsorption isotherm plot for mild steel corrosion in 1M HCl in presence of TPL leaves extract at different temperature
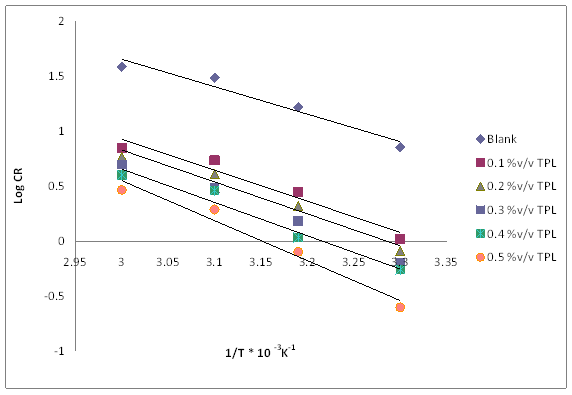
Figure 10. Arrhenius plot for mild steel corrosion in 1M HCl in the absence and presence of TPL leaves
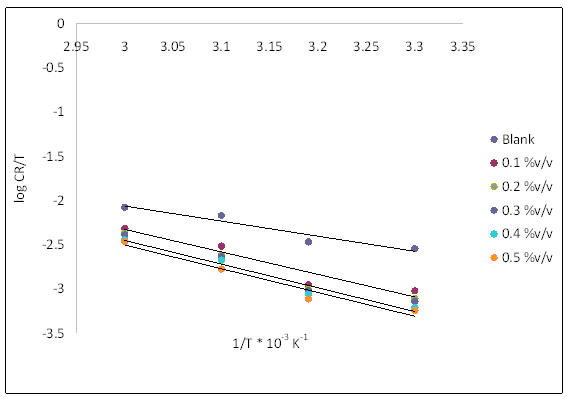
Figure 11. Transition state plot for mild steel corrosion in 1 M hydrochloric acid in the absence and presence of acid extract of TPL leaves
Discussion
Characterization of the extract and corrosion product
The FTIR spectra of the leaves extract of TPL and the dried corrosion adsorption product are presented in Figures 1 and 2.In both Figures the spectra show the presence of amino groups (-NH), phenolic hydroxyl groups (-OH), and carbonyl group (C-O). However, it is observed that the stretching frequencies of the OH, NH, and C=O groups shifted from 3425 to 3386.33 cm-1, 2360.00 to 2365.71 cm-1 and 1643.10 to 1630 cm-1, respectively. This indicates that the oxygen and nitrogen atoms of OH, C=O and NH groups have coordinated with Fe2+ resulting in the formation of Fe- TPL complex on the metal surface. The shift in stretching frequencies has been noted to suggest that the active phytochemical constituents present in the inhibitor molecules bind to the metal surface. They form a protective metal-inhibitor complex to reduce the further dissolution of the metal in the aggressive solution. The changes in the adsorption bands also suggest that the adsorption between the extract and mild steel takes place through these functional groups. Deeparani et al. [5] and Johnsirani et al. [6] also reported similar results.
Corrosion behaviour
Figure 3 shows the variation in mass loss of mild steel in 1M HCl in the absence and presence of different concentrations of the Tridax procumbens L leaves extract as a function of time. From the results, it is observed that there is a very significant reduction in mass loss with the addition of the extract. The mass loss increased with increase in immersion time but decreased with increase in the concentration of the extract. The mass loss trend is supported by the results from the potentiodynamic polarization curves and electrochemical parameters (Figure 4 and Table 1, respectively). From Figure 4, it is observed that corrosion cells containing higher concentrations of the TPL leave extract are the polarization curves shifted more to the left, indicative of relatively lower corrosion current density. Thus affirming that with increase in TPL leave extract (within the range utilized in this study), there appears to be improved protection of the mild steel substrates from corrosion attack by the 1M HCl medium. Table 1 shows the electrochemical parameters expressive in quantitative terms of the effect of increased concentration of the extract on the corrosion potential (Ecorr), current density (Icorr) and corrosion rates. The Icorr and corrosion rates are clearly observed to decrease with increase in the extract concentration. The increase in concentration of the extract retarded the dissolution of the mild steel due to the adsorption of the phytochemical constituents on the metal surface through Fe 2+,thereby creating a barrier between the metal and the acidic medium. This enhanced inhibition and resulted in a smaller mass Ioss compared to the uninhibited metal sample [7]. The SEM micrographs (Figure 5), confirm that a higher depth of corrosion occurred in the mild steel substrate which did not have the Tridaxprocumbens L leaves extract compared with the representative grade of substrates containing the extract. Well pronounced corrosion pits are observed in the substrate without the extract (Figure 5b) -the pits represent pitting corrosion caused by the aggressiveness of Cl- ions on the metal. The pits in the substrate immersed in the 1MHCl solution containing the extract (Figure 5c), are noted to be less visible indicating a lesser intensity of pitting corrosion. The results of the AAS analysis presented in Figure 6 was a confirmatory test performed to measure the concentrations of Fe2+ that diffused into the electrolyte solution after the weight loss test. It is observed from the graph that there is a significant reduction in the concentration of iron (II) ions in the acid solution containing the extract in comparison with the blank. The trend strongly supports the claim that the Tridax procumbens L leaves extract is responsible for the very high corrosion resistance observed from the research samples containing this extract.
Influence of concentration and temperature on the corrosion rate and inhibition efficiency of the extract
The variations in corrosion rate and inhibition efficiency of the Tridax prucumbens L leave extract with temperature are presented in Figures 7 and 8. From Figure 7, it is observed that the corrosion rate of the mild steel decreases with the concentration of the extract but increases with temperature. Increase in corrosion rate with temperature is normally associated with increase in the average kinetic energy of the reacting molecules [17].This notwithstanding, the corrosion rates were generally low for the solutions containing the extract irrespective of temperature. This fact is well appreciated from the inhibition efficiency data presented in Figure 8. It is evident from Figure 8 that inhibition efficiency decreases with increasing temperature. This may be due to increased rate of dissolution of mild steel as pointed out earlier and partial desorption of the inhibitor from the metal surface with temperature. The highest concentration of the extract, 0.5%v/v, gave the highest inhibition efficiency of 96.5 % at the temperature of 303K, which dropped to 92.4 % at 333K (4.25 % reduction in inhibition efficiency). In the case of the lowest TPL extract concentration of 0.1%v/v, the extract gave an inhibition efficiency of 85.43% at the temperature of 303K which dropped to 81.71 % at 333K (4.35 % reduction in inhibition efficiency). It should be noted that a decrease in inhibition efficiency with rise in temperature is frequently associated with physical adsorption mechanism [18]. This supports the reasoning that the phytochemical constituents of the TPL leaves extract were physically adsorbed on the surface of mild steel. Nevertheless, the high inhibition efficiencies obtained at the high temperature of 333K shows that TPL can still inhibit effectively at elevated temperatures.
Adsorption characteristics of TPL extract on mild steel in HCl solution
The plot of C/θ versus C is presented in Figure 9. It is observed from the plot that for all test temperatures, a linear fit of the data is obtained indicating that the adsorption of the inhibitor on the surface of mild steel is consistent with Langmuir isotherm and the slopes obtained are unity. The correlation coefficient (R2) of the adsorption isotherm data showed that Langmuir isotherm is best fitted into the experiment with R2 ranges from 0.9990 - 0.9997 for the extract and at different temperatures.
It should be noted that adsorption isotherms describe the adsorption mechanism and the interaction between metal surfaces and extract molecules. The simplest being the Langmuir isotherm, which is based on the assumption that all adsorption sites are equivalent and that particle binding occurs independently from nearby sites [19]. Also, surface coverage data play an important role in the assessment of inhibitor characteristics and are useful for fitting experimental data into adsorption isotherms which give an insight into the inhibition mechanism [1].
To further understand the adsorption behaviour of the TPL extract on the mild steel substrate, the activation energies in the absence and presence of TPL leaves extract were evaluated using Arrhenius equation [7]:
log(CR) = log(A) - Ea/(2.303∙R∙T) (4)
where CR is the corrosion rate, Ea is the apparent activation energy of the corrosion process, R is the general gas constant, and A is the Arrhenius pre-exponential factor. A plot of log of corrosion rate obtained by weight loss measurement versus 1/T produced a straight line for cases of absence and presence of the extract as shown in Figure 10.
The values of activation energy are presented in Table 2. The data show that the activation energy (Ea) of the corrosion of mild steel in 1M HCl solution in the presence of extract is higher (69.58kJ mol-1) than that in its absence (47.82kJ mol-1). The increase in the apparent activation energy for mild steel corrosion in inhibited solution is interpreted as physical adsorption [20]. This is in conformity with the findings that inhibition efficiency decreases with increasing temperature. More so, the values of the energy increases with concentration. This indicates that the energy barrier for the metal dissolution increases with concentration.
The enthalpy (∆Ha) and entropy (∆Sa) of activation of corrosion process were calculated from an alternative formulation of Arrhenius equation [7]:
log(CR/T) = log(R/(nh)) + ΔSa/(2.303∙R) - ΔHa/(2.303∙R∙T) (5)
where CR is the corrosion rate at Temperature T, R is the molar gas constant, n is Avogadro’s constant, and h is the Planck’s constant. A plot of log CR/T vs. 1/T yielded a straight line graph as shown in Figure 11, with a slope of (- ∆H/2.303R) and an intercept of (log(R/nh) + ∆S/2.303R). The values of ΔHa and ΔSa were calculated from the slope and intercept respectively and equally presented in Table 1. The results show that the enthalpy of activation values is all positive, which reflects the endothermic nature of the mild steel dissolution process [20]. More so, the enthalpy of activation in the presence of the extracts is greater than that obtained in its absence.
Conclusions
The corrosion behaviour and adsorption characteristics of varied concentrations of Tridax prucumbens Linn leave extract in 1M HCl solutions containing mild steel substrate was investigated in this study. From the analyses of the results obtained, the following conclusions can be made:
¸ The inhibition efficiency of the extract was very good as values within the range of 81 and 96.5 % were observed.
¸ The inhibition efficiency values increased with increase in the extract concentration but decreased with increase in temperature (although less than 5 % reduction was observed within the test temperature ranges).
¸ The adsorption process fitted perfectly with the Langmuir adsorption isotherm, and the effect of temperature revealed physical adsorption.
¸ Activation energy Ea was found to increase with increase in extract concentration which shows that the adsorbed organic matter provided a physical barrier to mass transfer, leading to reduction in corrosion rate.
¸ OH, NH, and CO groups were confirmed as the active phytochemical constituents in the extract responsible for the adsorption.
References
1. Alaneme K. K., Daramola Y.S., Olusegun S.J., Afolabi A.S., Corrosion inhibition and adsorption characteristics of rice husk extracts on mild steel immersed in 1M H2SO4 and HCl solutions, International Journal of Electrochemical Science, 2015, 10, p. 3553- 3567.
2. Belkhaouda M., Bammou L., Salghi R., Benali O., Zarrouk A., Ebenso E. E., Hammouti B., Avogado Nuts Extract (ANE): An efficient Inhibitor of C38 Steel Corrosion in Hydrochloric Acid, Journal of Materials and Environmental Science, 2013, 4 (6), p. 1042-1051.
3. Loto C. A., Joseph O. O., Loto R. T., Popoola A. P. I., Corrosion Inhibitive Behaviour of Camellia Sinensis on Aluminium Alloy in H2SO4, International Journal of Electrochemical Science, 2014, 9, p. 1221 – 1231.
4. Alaneme K. K., Olusegun S. J., Corrosion Inhibition Performance of Lignin Extract of Sun Flower (Tithonia Diversifolia) on Medium Carbon Low Alloy Steel Immersed in H2SO4 Solution, Leonardo Journal of Science, 2012, 20, p. 59-70.
5. Deepa R. P., Phetchiammal A., Nanthini T., Mariammal S., Selvaraj S., The Effect of Eugenia jambolana on Zinc in 1.0N Hydrochloric Acid Environment, IJGHC, 2013, 2 (3), p. 510-52.
6. Johnsirani V., Sathiyabama J., Rajendran S., Lydia Christy S. M., Jeyasundari J., The Effect of Eclipta Alba Leaves Extract on the Corrosion Inhibition Process of Carbon Steel in Sea Water, Portugaliae Electrochimica Acta, 2013, 31 (2), p. 95-106.
7. Alaneme K. K., Olusegun S. J., Adelowo O.T., Corrosion inhibition and adsorption mechanism studies of Hunteria umbellata seed husk extracts on mild steel immersed in acidic solutions, Alexandria Engineering Journal, 2015, http://dx.doi.org/10.1016/j.aej.2015.10.009
8. Shalabi K., Abdallah Y., Hassan H. M., Fouda A. S., Adsorption and Corrosion Inhibition of Atropa Belladonna Extract on Carbon Steel in 1 M HCl Solution, International Journal of Electrochemical Science, 2014, 9, p. 1468 – 1487.
9. Abubakar G., Ibrahim H., Apugo-Nwosu T. U., Mustafa M., Akuso S. A., Agboun D. T., Nwobi B. E., Ayilara S. I., Corrosion Inhibition Studies Of Ficus Abutilifolia on N-80 Oil Well Tubular Steel In 15% Hydrochloric Acid Solution, International Journal of Engineering and Computer Science, 2013, 2(8), p. 2364-2370.
10. Sheeja V. N., Subhashini S., PavettaIndica Bark as Corrosion Inhibitor in Mild Steel Corrosion in HCl and H2SO4 Medium: Adsorption and Thermodynamic Study, Chemical Science Transactions, 2014, 3 (1), p. 129-140.
11. Ankita J., Jain A., Tridax procumbens (l.): a weed with immense medicinal importance: A review, International Journal of Pharmacy and Bio Science, 2012, 3 (1), p. 544-552.
12. Sahoo M., Chand P. K., In vitro multiplication of a medicinal herb Tridax procumbens L. (Mexican Daisy, coat button): influence of explanting season, growth regulator synergy, culture passage and planting substrate, Phytomorphology, 1998, 48, p. 195 – 206.
13. Alaneme K. K., Alo A. W., Olusegun S.J., Corrosion Inhibitory Properties of Elephant Grass (pennisetum purperum) Extract: Effect on Mild Steel Corrosion in 1M HCl Solution, Alexandria Engineering Journal, (In Press).
14. Singh K., Ahirwar V., Acute and chronic toxicity study of Tridaxprocumbens on haemoglobin percent and blood sugar level of spraguedawley rats, IJPI’s Journal of Pharmacology and Toxicology, 2010, 1 (1), p.1-6.
15. ASTM G59-97, Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements, ASTM International, West Conshohocken, PA, 2014, www.astm.org.
16. Silverstein R. M., Bassler G. C., Morrill T. C., Spectrometric Identification of Organic Compounds, 4th edition, Wiley Publishers, New York, 1981.
17. Abd-El-Nabey B. A., Abdel-Gaber A. M., Said Ali M. El., Khamis E., El-Housseiny S., Cannabis Plant Extract as Inhibitor for the Corrosion of Nickel in 0.5 M H2SO4, International Journal of Electrochemical Science, 2012, 7, p. 11811 – 11826.
18. Ebenso E. E., Eddy N. O., Odiongenyi A. O., Corrosion Inhibitive properties and adsorption behaviour of ethanol extract of piper guinensis as green corrosion inhibitor for mild steel in H2SO4, African Journal of Pure and Applied Chemistry, 2008, 29(11), p. 107-115.
19. Khaled K. F., Hackerman N., Investigation of the inhibitive effect of ortho-substituted anilines on corrosion of iron in 1 M HCl solutions, Electrochimica Acta, 2003, 48, p. 2715 – 2723.
20. Singh A., Ahamad I., Yadav D. K., Singh V. K., Quraishi M. A., The effect of environmentally benign fruit extract of Shahjan( moringaoleifera ) on the corrosion of mild steel in hydrochloric acid solution, Chemical Engineering Communications, 2012, 199 (1), p. 63-77.