Engineering, Environment
Mineralogy and geotechnical characteristics of some pottery clay
Mujib Olamide ADEAGBO *, Samuel Akinlabi OLA, Olumide Oluwapelumi OJURI
Dept. of Civil and Environmental Engineering, Federal University of Technology, Akure, Nigeria
E-mail(s): adeagbolami@gmail.com; samuelola41@yahoo.com; ooojuri@futa.edu.ng
* Corresponding author, phone: 234 (0) 8182290386, 234 (0) 7085649750
Received: May 16, 2016 / Accepted: December 24, 2016 / Published: December 30, 2016
Abstract
The physical properties of soils, which are tremendously influenced by the active clay minerals in soil, are of great importance in geotechnical engineering. This paper investigates the clay-sized particles of the Igbara-Odo pottery clay, and compares results obtained with available data on the bulk sample, to determine their correlation and underline the dependence of the geotechnical properties of the bulk clay material on the clay-sized particles. The bulk clay sample consists of 52% sand-size particles, 21% silt and 27% clay. Analysis of the clay-sized particles and the bulk materials shows: specific gravity of 2.07 and 2.66, liquid limit of 91.0% and 33.0%, plastic limit of 27.5% and 14.3%, plasticity index of 63.5% and 18.7% and a linear shrinkage of 7.9% and 5.4%, for both clay-sized particles and bulk clay respectively. The activity value of the clay material (0.64) suggests the presence of Kaolinite and Ilite; and these were confirmed with X-Ray diffraction on the bulk sample and clay-sized particles. X-Ray diffraction patterns shows distinctive peaks which highlight the dominance of Kaolinite (with 8 peaks) in the pottery clay sample for both clay-sized particles and bulk material; while traces of other clay minerals like Illite and Halloysite and rock minerals like Mica, Feldspar and Chrysotile were also found. These results suggest that the clay possesses high viability in the manufacturing of ceramics, refractory bricks, paper, fertilizer and paint. The clay material can be used as a subgrade in road construction, since it possesses low swelling characteristics.
Keywords
Clay fraction; Geotechnical properties; Mineralogy; Pottery clay
Introduction
Common clay is the most widespread ceramic materials in the world [1]. Pottery is a prehistoric part of past pre-literate culture and therefore, much of the world’s history, and can be found among the artefacts of archaeology. The abundance of the clay minerals in Nigeria supports its rich and historic traditional pottery industry that dates from the Stone Age. Archaeological evidences from the ancient pottery areas of Nigeria such as Iwo-Eleru near Akure in Ondo State, Rop in Plateau state, Kagoro in Kaduna State and Afikpo in Ebonyi state proved that as far back as the late stone age, the occupants of these areas made productive used of clay for pottery [2]. In Ekiti State, pottery clays can be found in large quantity in some towns and villages which include Isan-Ekiti, Ara-Ekiti, Ilafon, Ire-Ekiti, Igbara-Odo, and Ado-Ekiti amongst others [3]. The durability quality of pottery has made pottery products survive for millennia at archaeology sites. Other properties important in the use of clay are the green and dry strength, fired strength, vitrification range, drying and fired shrinkage, fired colour and fired density [1, 4]. As the dependence of clay products performance on these properties have been highlighted, there is a need to investigate each clay soil, characterize it and determine the type of products such clay will be suitable to produce.
The Wenworth scale defines the clay grade as finer than 4µm [5]; which is used by many Engineers and Scientists whereas clay scientists generally consider 2µm as the upper limit of the clay size grade [6]. Clays are distinguished from other fine-grained soils by differences in size and mineralogy. Silts, which are fine-grained soils that do not include clay minerals, tend to have larger particle sizes than clays, but there is some overlap in both particle size and other physical properties, and there are many naturally occurring deposits which include silts and also clay. The distinction between silt and clay varies by discipline. Geologists and soil scientists usually consider the separation to occur at a particle size of 2 µm (clays being finer than silts), sedimentologists often use 4-5 μm, and colloid chemists use 1 μm [7]. Geotechnical Engineers distinguish between silts and clays based on the plasticity properties of the soil, as measured by the soils' Atterberg limits. ISO 14688 [8] grades clay particles as being smaller than 2 μm and silts larger.
Igbara-Odo pottery clay is classified as Ferruginous soil [9]. The bioclimatic nature of the environment available at Igbara-Odo allows easy weathering process as the tropical climate found there is characterised by intense rainfall, about 20-300C temperature etc. This also paves the way for important factors like leaching and abundance of vegetation which affect the nature of weathering products. The soils and vegetation are typical red-brown tropical ferruginous soils and rain forest with abundance of deciduous trees. The soils at the foot of upland areas are rich in pottery clay which are whitish creamy or dark-creamy in colour but poor in organic matter. The soil profile of Igbara-Odo and its environs displays distinct layers and the individual horizons differ from one another in their properties and composition. Paedogenesis rearranges the material within the soil profile, particularly by leaching of components from the top as rain water percolates through the soil [10] (Figure 4). Generally, the underlying soil at Igbara-Odo is Clay with the upper layers being either transported or residual sandy, loam or laterite.
Previous works have been carried out on the clay content of Nigerian Soils to determine the variation in properties such as a research into the geotechnical and geochemical properties of some clays occurring in Ilorin, Nigeria [11], a study of the Important Properties of Clay Content of Lateritic Soils for Engineering Project [12], a study of the Compositional, Geotechnical and Industrial Characteristics of Some Clay Bodies in Southern Nigeria [13], evaluation of the Geotechnical Properties of Ilesha East South-western Nigeria’s Lateritic Soil [14], investigation of the Relationship between Clay Content, Clay Type, and Shrinkage Properties of Soil Samples [15] as well as a research on the economic potentials of clay monerals in Nigeria [16]. However, extensive research has not been done on the primary ball clays of Ekiti state or on the clay fraction of pottery clays in Nigeria. Building on previous works on some local pottery clays [3], this study’s aim is to underline, with the aid of physical and chemical properties, the importance of the clay content of pottery clays on the characteristics of the pottery clay found in this region. The paper also analyses the engineering importance of the clay fraction of pottery clays by determining the strength characteristics, clay minerals present and other properties of pottery clay, with a view to investigate the clay’s potentials for engineering purposes in buildings and road constructions.
Material and Method
The study area
The study site is located at Igbara- Odo in Ekiti South-West Local Government Area of Ekiti State in South-Western Nigeria (figure 1).
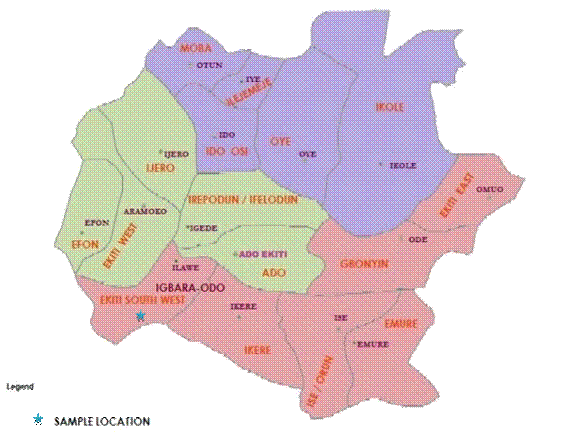
Figure 1. Map of Ekiti State showing the location of the sample
It is situated at the southern end of the Precambrian Basement Complex rocks of South-Western Nigeria [17] (as shown in figure 2), bounded by parallels 7°26¢N and 7°35¢N and Eastings 4°56¢E and 5°11¢E. Igbara-odo is endowed with scattered uplands (hills and mountains) and valleys, with a peak of 765m and troughs of 400m above the sea level (figure 3). Excavations have shown the presence of different types of clays within the top 10 metres of the soil profile, being overlaid by lateritic soil or sandy-loam in other places (figure 4). Samples for this research work were excavated from the forestland along the Igbara-Odo – Igbara-Oke Road when coming towards the Igbara-Odo Town.
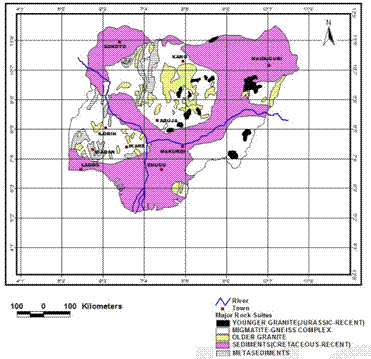
Figure 2. The Simplified Geological Map of Nigeria (from ref. [17])
![]()
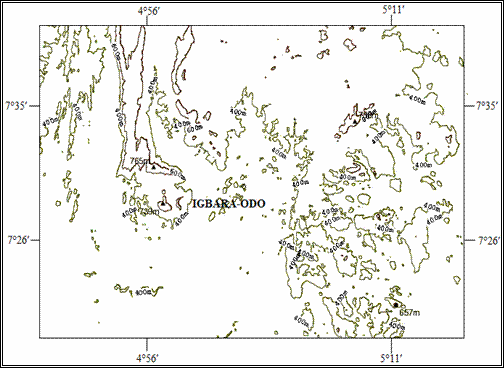
Figure 3. Topographical Map of Igbara-Odo
The site is located at the left side of the road towards Igbara-Odo (about 200m away from the centre-line of the roadway) and is just about 1km away from the Igbara-Odo – Igbara-Oke – Ikere-Ekiti – Igede-Ekiti junction (figure 5). The study site is situated at a longitude of 7.50251 °N and latitude 5.06258 °E and is about 450m above mid-sea level.
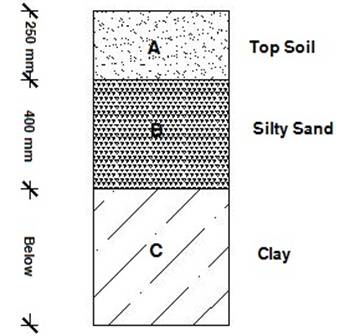
Figure 4. Soil Profile of Igbara-Odo (from ref. [10])
Figure 5. Map of Igbara-Odo showing location of study site
Sample collection and preparation
For detailed investigation and assessment of the clay samples in the study area, disturbed (bulk) samples were collected after excavating to a depth of about 2.0 m below the existing ground level. Considering the relative proportion of the clay-sized particle in the bulk sample, sufficient amount (about 120 kg) of the sample was packed in labelled sample bags and transported to the laboratory for tests and analysis. Thereafter, the collected samples were oven-dried (at 105 °C) for about 48 hours before been subjected to laboratory tests in accordance to BS 1377 standard for Soil Tests [18].
Hard lumps of the clay were manually crushed and dry-sieved through the No. 200 sieve. Thereafter, the sieved sample was dispersed in and thoroughly mixed with distilled water to properly saturate the sample in a mass ratio of 1:10 in a sedimentation tank. After about 8 hours, the top 10cm of the tank was siphoned off using a siphoning tube into clean plates, representing particles that are less than 2 µm in size. The mixture in the sedimentation tank was stirred thoroughly again, and allowed to stay for at least 8 hours again before another set of sample can be taken again. These collected samples were oven-dried in stainless plates and the resulting cake and sheets were collected and pulverised to obtain fine particles, in readiness for laboratory tests. Physical tests carried out on the prepared sample include specific gravity, Atterberg’s limits (Consistency Limit) tests, clay activity while the clay Mineralogical determination was done by X-Ray Analysis.
Experimental procedures
To determine the particle size composition of the bulk sample, dry-sieving analysis and hydrometer test (after wet-sieving through the No. 200 sieve) was done. Determination of the specific gravity of the clay-sized particles was achieved by using the density bottle technique.
The Atterberg’s Limit is used to determine the empirical water content boundary of samples i.e. the liquid, plastic, semi-solid and solid stages [19]. The liquid limit is defined as the moisture content at which a standard groove cut through a remolded sample will close over a distance of ½ inches (13 mm) at 25 blows of the Casagrande apparatus’ cup. The Plastic limit (transition between the plastic and semisolid state) is defined as the moisture content at which a thread of soil just crumbles when it is carefully rolled out by hand to a diameter of about 3mm. The numerical difference between the liquid limit and the plastic limit of a soil is known as the plasticity index, which is the range of moisture content over which the soil is in plastic condition. The shrinkage limit was determined by noting the percentage decrease in length when a Soil sample paste at the approximate liquid limit is placed inside the shrinkage mould and oven-dried; while the the Plasticity Index is evaluated by the difference between the liquid limit and the plastic limit (PI = LL- PL). The activity of the clay particles was then determined by the ratio of the plasticity index to the percentage of clay fraction present in the used sample, in order to estimate the dominant clay minerals [20].
Identification of the clay and non-clay minerals (rock minerals) was done by the X-ray diffraction (XRD) technique. Qualitative analysis was carried out on the pulverised prepared samples (with particle size <2 microns) using the Air dried preferred orientation. A Pan Analytical X-ray diffractometer equipped with Ni-filtered Cu-Kα radiation generated at 40 kV and 55 mA, a goniometer speed of 2°/min and chart speed of 1200 mm/h; which ran for about 2 hours. Identification of clay minerals was according to the d-value obtained from peak values of the X-Ray patterns by the Bragg’s Law (Eq. 1).
n·λ = 2·dhkl·sin(θ) (1)
where ![]() is
the wavelength of the X-ray (measured in Ångströms, i.e. 10-10
metres), n is the integral number of wavelengths, which is
assumed as 1, dhkl is the distance between two parallel
planes of atoms and q is the angle of incidence of monochromatic parallel X-ray beams.
is
the wavelength of the X-ray (measured in Ångströms, i.e. 10-10
metres), n is the integral number of wavelengths, which is
assumed as 1, dhkl is the distance between two parallel
planes of atoms and q is the angle of incidence of monochromatic parallel X-ray beams.
The standard tables were used in estimating the minerals are the X-Ray powder data for minerals [21]. The assumed wavelength (λ) value for the Cu-Kα radiation is 1.5418 Å.
Results
Grain Size Distribution and Specific gravity
The percentage grain sizes distribution of the bulk sample is presented in the particle size distribution curve below (Figure 6). The bulk sample of the pottery clay contains high clay-sized particle (27%), the silt fraction (21%) and sand particles content of about 52%. For the clay-sized particles, a specific gravity of 2.07 was obtained while the bulk sample has a specific gravity of 2.66.
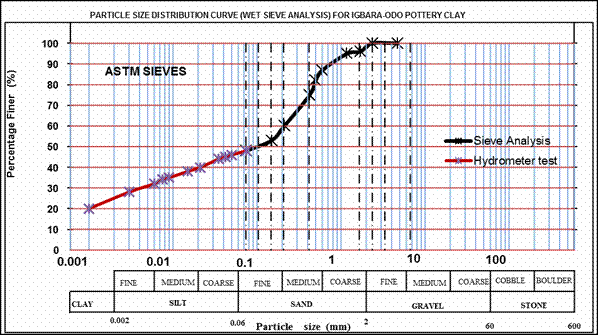
Figure 6. Particle Size Distribution Curve for the Bulk Sample
Atterberg’s Limit
For the clay-sized particles, a liquid limit of 91% (figure 7), plastic limit of 27.5%, and Shrinkage of 7.9% were obtained from laboratory tests. With these data, a plasticity index of 63.5% was derived. Also, to a clay activity value (ratio of plasticity index to the percentage clay) of 0.64 was arrived. Table 1 summarizes the physical characteristics of the pottery clay samples.
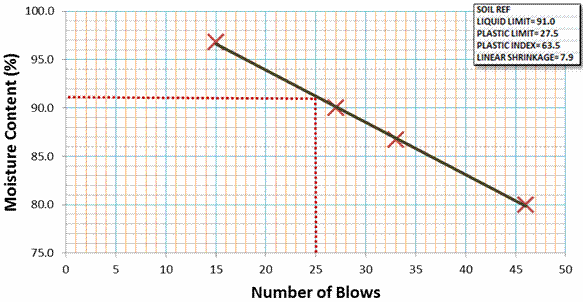
Figure 7. Graph of Liquid Limit Test Carried out on the Clay-sized Particles
Table 1. Summary of the Pottery clay characteristics
Sample properties |
|
sample type |
||
|
|
bulk sample* |
clay particles |
||
|
Particle size distribution, % |
|
|
|
|
|
Sand |
|
52.00 |
|
|
|
Silt |
|
21.00 |
|
|
|
Clay |
|
27.00 |
100.00 |
|
|
Fines fraction |
|
48.00 |
100.00 |
|
|
Specific gravity, Gs |
|
2.66 |
2.07 |
|
|
Atterberg limit, % |
|
|
|
|
|
Liquid limit, L.L |
|
33.00 |
91.00 |
|
|
Plastic limit, P.L |
|
14.30 |
27.50 |
|
|
Plasticity index, P.I |
|
18.70 |
63.50 |
|
|
Linear Shrinkage |
|
5.40 |
7.90 |
|
|
Clay Activity |
|
|
0.64 |
|
*Source for bulk sample data: [3]
Mineralogical characteristics
The clay minerals in the sample were identified from the oriented and random powder diffraction pattern following classification methods using the d-value obtained from peak values of the X-Ray patterns [22]. The X-Ray pattern obtained from the diffractometer using the air dried preferred orientations are presented in Figures 8 and 9.
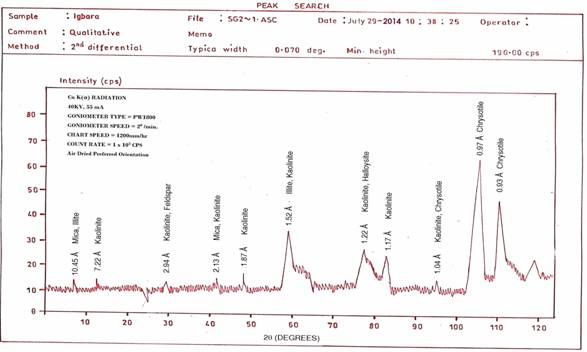
Figure 8. X-Ray Diffraction Pattern of Igbara-Odo Pottery Clay (Clay-size Particles)
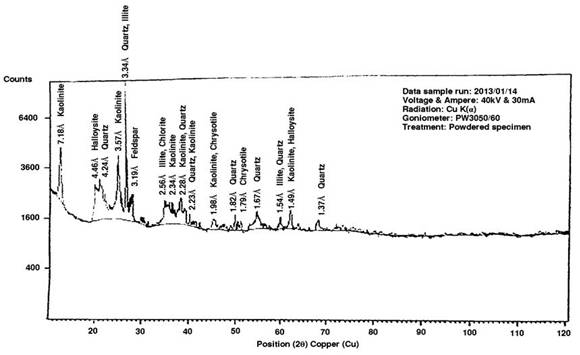
Figure 9. X-Ray Diffraction Pattern of the Bulk Sample [3]
From equation 1, the d-value (in Å) is given by equation 2 if λ = 1.5418Å and n = 1:
![]() (2)
(2)
The d-values (in Angstroms) at each peak in the patterns were evaluated by substituting the corresponding values of q (i.e. dividing 2q by 2) in equation 2, as shown in tables 2 and 3; for the clay-sized sample and bulk sample respectively. The corresponding intensities (read from the diffraction pattern) are also included in the tables. The d-values were then matched to the associated minerals as provided on the standard tables for classification.
Table 2. Mineralogical analysis of Igbara-Odo pottery clay (< 2 microns particles)
|
Peak No. |
2θ (°) |
d-value (Å) |
Intensity (cps) |
Minerals |
|
1 |
8.46 |
10.4514 |
14 |
Mica, Illite |
|
2 |
12.26 |
7.2192 |
15 |
Kaolinite |
|
3 |
30.38 |
2.9421 |
10 |
Kaolinite, Feldspar |
|
4 |
42.44 |
2.1299 |
12 |
Mica, Kaolinite |
|
5 |
48.78 |
1.8668 |
12 |
Kaolinite |
|
6 |
60.82 |
1.5230 |
33 |
Illite, Kaolinite |
|
7 |
78.02 |
1.2247 |
26 |
Kaolinite, Halloysite |
|
8 |
82.49 |
1.1693 |
24 |
Kaolinite |
|
9 |
95.24 |
1.0436 |
13 |
Kaolinite, Chrysotile |
|
10 |
105.26 |
0.9700 |
60 |
Chrysotile |
|
11 |
112.82 |
0.9254 |
40 |
Chrysotile |
Table 3. Mineralogical analysis of Igbara-Odo pottery clay bulk sample [3]
|
Peak no. |
2θ (°) |
d-spacing (Å) |
Intensity (cts) |
Rel. Int. (%) |
Minerals |
|
1 |
12.33 |
7.1789 |
4601.48 |
34.43 |
Kaolinite |
|
2 |
19.89 |
4.4631 |
1498.52 |
17.79 |
Kaolinite, Halloysite |
|
3 |
20.92 |
4.2462 |
1550.67 |
20.76 |
Kaolinite |
|
4 |
24.91 |
3.5749 |
3222.78 |
36.45 |
Kaolinite |
|
5 |
26.67 |
3.3421 |
7469.30 |
100.00 |
Illite, Quartz |
|
6 |
27.96 |
3.1917 |
3980.98 |
13.13 |
Feldspar |
|
7 |
34.99 |
2.5647 |
2823.66 |
11.03 |
Kaolinite, Halloysite, Illite |
|
8 |
38.44 |
2.3417 |
1879.85 |
11.78 |
Kaolinite |
|
9 |
39.47 |
2.2829 |
1477.00 |
6.39 |
Kaolinite, Quartz, Chlorite |
|
10 |
40.33 |
2.2366 |
1366.67 |
4.91 |
Kaolinite, Halloysite, Quartz |
|
11 |
45.63 |
1.9881 |
1283.44 |
3.79 |
Kaolinite, Quartz, Chrysotile |
|
12 |
50.14 |
1.8195 |
1054.77 |
6.09 |
Kaolinite, Quartz |
|
13 |
50.74 |
1.7992 |
1061.45 |
3.50 |
Kaolinite, Chrysotile |
|
14 |
51.41 |
1.7774 |
1286.49 |
3.84 |
Kaolinite |
|
15 |
55.02 |
1.6691 |
1983.85 |
6.48 |
Kaolinite, Halloysite |
|
16 |
60.02 |
1.5415 |
1373.43 |
5.00 |
Kaolinite, Illite, Quartz |
|
17 |
62.26 |
1.4913 |
1702.32 |
8.06 |
Kaolinite |
|
18 |
63.96 |
1.4557 |
1964.45 |
2.20 |
Kaolinite, Quartz |
|
19 |
68.11 |
1.3755 |
1751.71 |
3.37 |
Kaolinite, Quartz, Chrysotile |
Table 4 provides a comparison between the minerals found in the samples (bulk and < 2 microns).
Table 4. Comparison of the mineralogical content of clay-sized particles and bulk sample
|
< 2 MICRONS PARTICLES† |
|
BULK SAMPLE†* |
|
|
Kaolinite |
|
Kaolinite |
|
|
Illite |
|
Illite |
|
|
Feldspar |
|
Halloysite |
|
|
Mica |
|
Feldspar |
|
|
Chrysotile |
|
Mica |
|
|
Halloysite |
|
Chlorite |
|
|
|
|
Quartz |
|
|
|
|
Chrysotile |
|
*Source: [3]; † - in descending order of intensity value
Discussions
Physical Characteristics
The percentage grain sizes distribution of the sample clearly shows that the sample is consisted mainly of fine particles, which is a characteristics of typical clays used in pottery. The silt clay ratio is generally above 0.15. Suitable materials used in pottery in Nigeria generally have about 38% to 54% passing the sieve No. 200 [3].
For the clay-sized particles, a specific gravity of 2.07 is obtained in contrast to 2.66 obtained for the bulk value. The reduction in the value of specific gravity obtained from the laboratory tests carried out compared to that obtained from the bulk sample indicates that the clay-sized particles has lesser proportion of mass in bulk clay sample, and that the sand part; composed mostly of quartz contributes to the mass of the bulk pottery clay sample. Thus, the sand particles, which account for half of the total mass has more influence on the rigidity and soundness of the clay sample.
The high value gotten for the liquid limit (91%) gives accommodation for a Plasticity Index of 63.5%, compared to 33% and 18.7% respectively for the bulk sample. The increased surface area available for hydration reaction and size of the particles allows a dipole to be formed, which attract water molecules and absorb them. This led to particles having sizes less than 2 microns having a greater liquid limit and Plasticity Index. Owing to the large surface area to thickness ratio, much water is needed to be absorbed into the skeleton before flow can begin [23]. The sample also possesses high plasticity, judging from experimental values (14.3% and 27.5% for the bulk sample and clay particles respectively). The high value of the Plasticity Index (63.5%) for the clay-sized particles also indicates that the plastic nature of the sample can be maintained for large moisture content variations. Also, the green and dry strength properties which are of importance in clay products are closely related to the plasticity of the clay.
The fireabilty property of the pottery clay (as a suitable ceramic material) in the two states was indicated by the low values of Shrinkage observed (7.9% for clay-sized particles and 5.4% for bulk sample). In essence, the clay-sized particles possess more shrinkage ability than the bulk sample because the particles in the clay-sized sample are more plastic. Materials moulded with the sample in either cases (bulk form or clay-sized) thus, undergo insignificant dimensional changes between their wet state and dried state; which is characteristic of Kaolinite clays [9]. The clay activity value of 0.64 (which is less than 0.75) confirms that the clay sample is an inactive clay and predicts that it is likely to contain kaolinite as its major clay mineral [20].
Mineralogical characteristics
The mineralogy of materials influences the performance and characteristics of such materials; and the mineralogical standard presented by a clay sample can maximize its use for a particular application [24]. From the mineralogy analysis table presented, it can be deduced that the absence of quartz in the particles used in this study, as opposed to the result obtained with the bulk sample, was because only the clay-sized particles were used for the research. Sedimentation process removes the sand and silty content of the bulk sample and only rock minerals like mica, feldspar and chrysotile (which are residues of formation process of clay minerals) were detected in addition to the clay minerals. The clay minerals detected in the sample in the two states (i.e. bulk sample and < 2 microns particles) are the same, which is mainly of the Kaolin group (Kaolinite and Halloysite), with traces of Illite.
Clays rich in kaolinite have lesser plasticity compared to clays rich in other clay minerals, owing to the hydrogen bonds between the structure sheets [1]. Kaolinites are very stable with strong structures (are composed of one tetrahedral sheet linked to an octahedral sheet, being classified as 1:1 type layer silicates; one consists of tetrahedral oxygens and the other of OH- ions belonging to the octahedral sheet [25]). They absorb little water and show relatively low swelling and shrinkage reactions to variations in Moisture content, thus making such clay ideal for engineering purposes [23]. Clay mineral types also affect the rate of drying of clay products. Vitrification or glass forming property of clays, which is important in the structural use of clay, is affected by clay mineralogy as Clays with minerals like Montmorillonites shows earlier vitrification during firing than kaolin [26], [27].
Conclusions
In the assessment of the geotechnical properties of the Igbara-Odo pottery clay, test results show that the engineering characteristics of the clay is more pronounced in the clay fraction particles (i.e. particles less than 2 microns in size) compared to that of the bulk sample. On this note, it can be said that the engineering properties of pottery clays; and residual clays in general, depends mostly on the properties of their clay-sized particles present therein. For example, the soil displays an increase in liquid limit, a corresponding increase in plasticity index and a slight decrease in the plastic limit.
The results obtained from the mineralogical analysis carried out by the X-Ray Diffraction method shows that the clay-sized particles are composed majorly of kaolinite with traces of illite, halloysite and rock minerals (mica, feldspar and chrysotile) and that the sand and silt content of the bulk sample contains quartz.
The abundance of kaolinite suggests that the clay material is of good dry strength, moderate plasticity, good drying characteristics, good fireability properties, less shrinkage and lesser vitrification makes the Igbara-odo pottery clay ideal for structural use in making clay products like clay bricks, tiles, ceramics products and several other engineering uses.
The absence of minerals with inter-crystal swelling characteristics, and other engineering strength parameters as carried out also suggest that the clay strata of Igbara-Odo can be used as good subgrade and sub-base materials in road construction projects.
Acknowledgements
The authors would also like to express their profound gratitude to the laboratory staff at the FUTA/Kure Geotechnical Laboratory, Civil and Environmental Engineering Department, Federal University of Technology, Akure, Nigeria for the sample preparation and testing.
We also appreciate Mr Sam Oje of Geology Laboratory, University of Ibadan, Ibadan, Nigeria for his help on the X-Ray diffraction analysis.
References
1. Murray H. H., Applied Clay Mineralogy: Occurences, Processing and Application of Kaolins, Bentonites, Palygorskite-Sepiolite, and common clay, Amsterdam, Elsevier Scientific Publishing Company, 2007.
2. Fatunsin A. K., Yoruba Pottery, National Commission for Museums and Monuments. Lagos, 1992, p. 17-19.
3. Adebayo F. O., Comparative analysis of geotechnical properties of selected pottery clays in Ekiti and Kogi states in Nigeria, M.Eng. Thesis, Department of Civil Engineering, Federal University of Technology, Akure, Nigeria, 2013.
4. Coroado et al., Clays from Vila Nova da Rainha (Portugal): Appraisal of their relevant properties in order to be used in construction ceramics. Acta Geodynamica et Geomaterialia, 2010, 7 (2), p. 189-200.
5. Wenworth C. K., A scale of grade and class terms for clastic sediments. Journal of Geology, 1922, 30, p. 377-392.
6. Ola S.A (Ed.), Essentials of geotechnical engineering, Ibadan, University Press Limited, 2013.
7. Guggenheim S., Martin R. T., Definition of clay and clay mineral, Journal report of the AIPEA nomenclature and CMS nomenclature committees, Clays and Clay Minerals, 1995, 43 (2), p. 255-256.
8. ISO 14688-1, Geotechnical Investigation and testing- Identification and classification of soil, International Standard, 2002, p. 12
9. Olokode O. S., Aiyedun P. O., Mineralogical Characteristics of Natural Kaolins from Abeokuta, South-West Nigeria. The Pacific Journal of Science and Technology, 2011, 12(2), p. 558-565.
10. Olajide A., A study of the erosion activities at Igbara-Odo, Ekiti, B.Tech. Thesis, Department of Applied Geology, Federal University of Technology, Akure, Nigeria, 1998.
11. Ogunsanwo O., Agbasi U., Geotechnical and geochemical properties of some clays occurring in Ilorin, Nigeria and the environmental applications of their mode of exploitation, 7th International IAEG (International Association for Geology and the Environment) Congress, 1994.
12. Adebisi N. O., Adeyemi G. O., Oluwafemi O. S., Songca S. P., Important Properties of Clay Content of Lateritic Soils for Engineering Project, Journal of Geography and Geology, 2013, 5(2), p. 99-115.
13. Onyeobi T. U. S., Imeokparia E. G., Ilegieuno O. A., Egbuniwe I. G., Compositional, Geotechnical and Industrial Characteristics of Some Clay Bodies in Southern Nigeria, Journal of Geography and Geology, 2013, 5(2), p. 73-84.
14. Bello A. A., Adegoke C. W., Evaluation of Geotechnical Properties of Ilesha East Southwest Nigeria’s Lateritic Soil, Pacific Journal of Science and Technology, 2010, 11(2), p. 617-624.
15. Boivin P., Garnier P., Tessier D., Relationship between Clay Content, Clay Type, and Shrinkage Properties of Soil Samples, Soil Science Society of America Journal, 2004, 68, p. 1145-1153.
16. Adelabu, O., Kashim I., Clay mineral: A case study of its potentialities in selected parts of Kaduna State of Nigeria. Proceeding of International Conference on Education and Management Technology (ICEMT), 2010, p. 655-659.
17. Oyediran I. A., Durojaiye H. F., Variability in the Geotechnical properties of some residual clay soils from south-western Nigeria, International Journal of Scientific & Engineering Research, 2011, 2(9), p. 235-240.
18. BS 1377-2, Methods of test for soils for civil engineering purposes, Classification tests, British Standard, 1990.
19. ASTM D-4318, Standard test method for liquid limit, plastic limit and plasticity index of soils, American Standard, 2010.
20. Skempton A. W., Selected papers on soil mechanics, United States, ICE Publishing, 1984.
21. Mitchell J. K., Fundamentals of Soil Behaviour, New York, John Wiley and Sons, 1976.
22. Brown G., Brindley G. W., Crystal structures of clay minerals and their X-ray identification, Mineralogical society monograph, 1980, 5, p. 495
23. Adelabu S. O., Documentation, Application and Utilisation of Clay Minerals in Kaduna State (Nigeria), Clay Minerals in Nature – Their Characterization, Modification and Application, 2012, p. 3-20.
24. Silva-Valenzuela M. G., Matos C. M., Shah L. A., Carvalho F. M. S., Sayeg I. J., Valenzuela-Diaz F. R, Engineering Properties of Kaolinitic Clay with Potencial Use in Drugs and Cosmetics, International Journal of Modern Engineering Research (IJMER), 2013, 3(1), p. 163-165.
25. Pohl W. L., Economic geology: principles and practice: metals, minerals, coal and hydrocarbons – introduction to formation and sustainable exploitation of mineral deposits, Chichester, West Sussex, Wiley-Blackwell, 2011, p. 321.
26. Grim R. E., Clay mineralogy, New York, Mc-Graw Hill, 1968, p. 596.
27. Yanik G., Ceylantekin R., Taşçi E., Özçay Ü., The Şahin village (Kütahya, Turkey) clay deposit and its possible utilization, Clay Minerals, 2012, 47(1), p. 1-10.