Engineering, Environment
The impact of hydroxypropylated Cassava starch substitution in feed on growth, nutrition, biochemical and toxicological parameters in rat
Sarah Onyenibe NWOZO1*, Louis Mahakwe NWOKOCHA2, Freda ITIGBRI1, Zainab JIMOH1
1Nutritional and Industrial Research Laboratories, Department of Biochemistry, Faculty of
Basic Medical Sciences, College of Medicine, University of Ibadan, Ibadan, Nigeria
2Industrial Chemistry Unit, Chemistry Department, Faculty of Science, University of Ibadan,
Ibadan, Nigeria
*Corresponding Authors email: sonwozo@yahoo.com; onyenibe.nwozo@mail.ui.edu.ng
Received: October 25, 2016 / Accepted: June 15, 2017 / Published: June 30, 2017
Abstract
Experimental diets (0%, 25%, 50% and 100%) using hydroxypropylated cassava starch (HPCS), was evaluated in rat model for one month, growth, antioxidant status, tissue histology and organ toxicity were investigated relative to animals on regular rat chow. Proximate analysis of HPCS compounded-feed showed protein, fibre and ash contents were lower compared to control feed; rats on HPCS had reduced feed-intake, lowered body-weight and organ-weights compared to control. HPCS feeding decreased significantly (P<0.05) urea and protein levels while creatinine and glucose-6-phosphate dehydrogenase (G6DP) increased significantly (P<0.05) in rats on 25% and 50% HPCS feeds compared to both control rats and 0% HPCS. Furthermore, the activity of ALT and ALP increased significantly (P<0.05), while AST significantly (P<0.05) decreased rats on 25%, 50% and 100% HPCS compared to control and 0% HPCS. HPCS feeding in rats elicited increased oxidative stress and lipid peroxidation as liver catalase (CAT), superoxide dismutase (SOD) and malonyl dialdehyde (MDA) were significantly (P<0.05) elevated compared to control and NCS. However, only CAT and SOD levels increased in the kidney of rats on HPCS but not MDA. Only rats on 100% HPCS diet, showed centrilobular fatty degeneration and congested veins in the liver histology while animals on other treatment groups had no lesions in both kidney and liver tissues. These results indicate that HPCS may have possible nephrotoxic, hepatotoxic and oxidative effects at different levels of modification and native cassava starch (NCS) or (0%HPCS) should be used with caution for proper growth of rats compared to commercial rat-chow.
Keywords
Feed-intake; Growth; Toxicity; Normal rat chow; NCS; HPCS
Introduction
Starch is one of the most important food ingredients possessing value added attributes for innumerable industrial applications especially when modified as well [1]. Starch is commonly stored in cereals (maize, guinea corn, millet, sorghum, rice) and in roots and tubers (irish/sweet potato, yam, cocoyam and cassava/tapioca). The chemical modification of starch may affect the rate and extent of its digestion in the small intestine [2]. It has been stated that the oxidation or dextrinization of starch, its substitution with hydroxypropyl, acetyl or octenyl succinate groups as well as crosslinking diminish its digestibility [2,3]. Hence, nutritionally starches subjected to chemical modification are acknowledged as resistant starches type 4 (RS 4).
Cassava starch chemical modification is mostly by the reactions of hydroxyl groups on the glucose ring moiety which could be esterification, etherification or oxidation [4]. Cyanogenic glycosides are present in raw unprocessed cassava and it could be contain broken down to produce hydrogen cyanide which can cause both acute and chronic toxicity in humans [5]. Hydrogen cyanide inactivates the enzyme cytochrome oxidase in the mitochondria by binding to the Fe3+/Fe2+ and this could cause a decrease in the oxygen utilization of the tissues. Cyanide causes an increase in blood glucose and lactic acid levels but ATP/ADP ratio decreases thus indicating a shift from aerobic to anaerobic metabolism. Processing of cassava such as peeling, slicing, fermentation and cooking helps to reduce both cyanogenic glycosides and hydrogen cyanide significantly and this reduces potential health risk on consumption. Cyanide ion can inhibit several iron, copper or molybdenum metalloenzymes as well as enzymes which contain Schiff base intermediates such as 2-keto-4-hydroxyglutarate aldolase. In female Albino fed raw cassava diet, haemoglobin concentration, packed cell volume, total serum protein concentration and T4 concentrations decreased while serum thiocyanate levels increased compared to control group [6].
The objective of this study is to evaluate the biochemical and toxicological effects of hydroxypropylated cassava starch (HPCS) and native cassava starch (NCS) which is from a much cheaper carbohydrate source in rat feeding experiment and evaluate its impact on growth and markers of tissue toxicity compared to control rat on commercial chow prepared from maize flour. These would contribute to application and safety issues of both NCS and HPCS in animal feeding and probable the use of either NCS or HPCS as substitute for carbohydrate in feed formulations.
List of abbreviations
NCS (native cassava starch), same as 0% hydroxypropylated starch; HPCS (hydroxypropylated cassava starch)
Material and method
Sample collection
Cassava starch sold for dietary purposes in the Gate market, Ibadan North Central Local Government Area was purchased: samples were air dried and packed into nylon bags until needed for feed formulation. HPCS (7%) was given to us from Industrial Chemistry laboratory, Chemistry Department, Faculty of Science, University of Ibadan, Ibadan already prepared and percentage hydroxypropylation determined.
Experimental Animals
Thirty male Wistar rats weighing between 93-110g were purchased from the Animal house of the Faculty of Veterinary Medicine, University of Ibadan, Ibadan, Nigeria. The rats were allowed to acclimatize for 1 week before they were randomly distributed into five groups of six animals. The animals were under standard conditions of temperature and humidity in the Animal house in Biochemistry Department, University of Ibadan. The animals in the control group were fed with normal laboratory rat feed purchased from Ladokun Feeds, Mokola, Ibadan and water was supplied ad libitum, while the others had formulated feed using native or hydroxylpropylated (HPCS) starch depending on the group. Protocol for this study and animal handling was approved by the Animal Ethics Board, Faculty of Veterinary Medicine, University of Ibadan.
Feed Formulation
The experimental diet was prepared as shown below in table 1:
Table 1. Concentration of HPCS
|
Components |
Concentrations of HPCS |
|||
|
0% |
25% |
50% |
100% |
|
|
Soy beans flour |
27 |
27 |
27 |
27 |
|
NCS |
59 |
44.25 |
29.5 |
- |
|
7% HPCS |
- |
14.7 |
29.5 |
59 |
|
Vegetable oil |
10 |
10 |
10 |
10 |
|
Vitamin mix |
4 |
4 |
4 |
4 |
The ingredients were thoroughly mixed, pelletized for easy handling by the animals, oven dried at 50°C until properly dried and stored in properly labelled air tight bags to prevent microbial spoilage. Feeds with varying of HPCS were compounded and were fed to rats for four weeks’ duration of the study.
Proximate analysis of diet
The proximate analysis on the NCS and HPCS feed was done using methods described by AOAC (1990) [7].
Sample Collection
The animals were fed for four weeks and sacrificed by cervical dislocation under ether anaesthesia. Blood samples were collected into anticoagulant bottles for plasma and non-anticoagulant tubes. Samples in anticoagulant were centrifuged at 3,500 rpm and plasma was collected into labelled Eppendorf tubes for mineral analysis. Blood samples collected into tubes without anticoagulant were left to stand for 1 hr before centrifugation at 3,500 rpm for 15minutes to obtain serum. The sera were decanted into tubes and stored at -80ºC in the deep freezer. The liver and kidney were washed, dried, weighed and stored in ice-cold 1.15% KCl buffer until needed. These were homogenized in ice-cold 0.1MTris buffer pH 7.4 using Potter-Elvehjem type homogenizer. The homogenates were centrifuged at 10,000 rpm at ≤ 40C for 15 minutes using Mistral 3000i cold centrifuge and the supernatants containing the liver and kidney microsomes were collected and stored at -800C in deep freezer until needed. Small bits of liver and kidney tissue was cut, washed to remove blood stains dried using filter paper, stored in a 40% buffered formaldehyde (formalin) solution before sending it to Veterinary Anatomy Department, University of Ibadan for tissue histology.
Biochemical assays
Serum creatinine and urea levels were determined using Randox kit. Glucose-6-phosphate dehydrogenase in liver homogenate was estimated using enzymatic colourimetric method. The activity of catalase in tissue homogenate was determined spectrophotometrically. Briefly, 1ml of sample was mixed with 49ml distilled H2O to give 1: 50 dilution of the sample. The assay mixture contained 4ml of H2O2 solution (800µmoles) and 5ml of phosphate buffer in a 10ml flat bottom flask. 1ml of properly diluted enzyme preparation was rapidly mixed with the reaction mixture by a gentle swirling motion. The reaction was run at room temperature. A 1ml portion of the reaction mixture was withdrawn and blown into 2 ml dichromate/acetic acid reagent at 60 seconds’ intervals. The mononuclear velocity constant, K, for the decomposition of H2O2 by catalase was determined using the equation for a first-order reaction: K= 1/t log S0/S
Where S0 is the initial concentration of H2O2 and S is the concentration of the peroxide at t min. The level of SOD activity, measuring thiobarbituric acid reactive substances (TBARS) according whereas earlier described by Nwozo et al., 2012 [8]. Aspartate, alanine aminotransferase and alkaline phosphatase activities were determined using Randox assay kit.
Statistical analysis
Results were expressed as the mean ± S.D. (n = 6). A one- way analysis of variance (ANOVA) was used for the data analysis. Significant differences among groups were determined using students t-test at p value less than or equal to 0.05.
Results and discussion
Starches from different botanical sources such as potatoes, wheat, rice and other foods, vary in colour and physiochemical characteristic. Native, unmodified starches tend to have limited applications in the food industry especially at extreme temperatures and pH ranges. In general, native starches produce weak-bodied, cohesive, rubbery pastes when heated and undesirable gels when the pastes are cooled. Thus, food manufacturers generally prefer starches with better behavioural characteristic than those provided by native starches which can be achieved by various modifications either by chemical or enzymatic methods. Modified starch is a food additive which is prepared by treating starch or starch granules, causing the starch to be partially degraded [9]. The purposes of this modification are to enhance its properties particularly in specific applications such as to improve the water holding capacity, heat resistant behaviour, reinforce its binding, minimize caking and improve thickening, hence making it possible for it to be useful as fat replacer, thickener etc [1011].
Feed composition, intake and growth parameters in rats fed varying percentages of HPCS diet
Feed composition has previously been shown and proximate analysis of compounded feed using HPCS and NCS are shown on Table 2.
Table 2. Proximate composition of both control diet (normal rat chow) and test diet
|
Components |
Commercial Rat Chow (Control feed) |
100% HPCS feed |
|
Moisture (%) |
8.830 |
8.470 |
|
Fat (%) |
4.880 |
7.650 |
|
Crude fibre (%) |
9.220 |
4.360 |
|
Ash (%) |
7.380 |
6.400 |
|
Protein (%) |
18.390 |
9.630 |
|
Carbohydrate (%) |
51.300 |
63.490 |
100% HPCS compounded feed had approximately 50% less protein, lower fibre and ash content compared to control diet. Test diet equally had higher carbohydrate and fat contents compared to commercially sold rat chow (Ladokun feed).
Weekly feed intake data are shown on Figure 1 with the control rats having the highest intake especially in the first week and the same group had recorded average intake for four weeks of 94.50±19.41. All test animals had their highest feed intake in the first week except those on 25% HPCS whose highest feed intake was in the second week. Feed intake gradually decreased over time in all feeding groups, average values from control, 0, 25, 50 and 100% HPCS were 94.50±19.41, 68.29±19.32, 66.85±10.89, 79.55±18.77 and 70.89±13.70; with that of 25% HPCS been statistically significantly decreased compared to control.
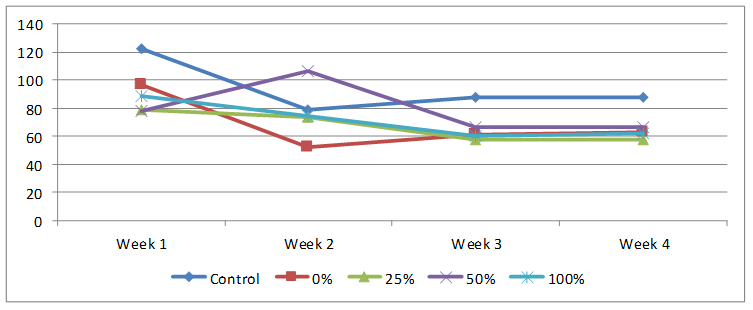
Figure 1. Weekly feed intake of rats on normal and test diet using different percentages HPCS
Changes in total body weights and organs are shown in Figures 2 and 3 respectively. Initial body weight was statistically not different (93-110) g but the final bodyweights after ingesting varying percentages of HPCS for four weeks were significantly decreased compared to control. 100% HPCS was significantly decreased compared to both control and 0% HPCS groups. Control rats had highest weight gain of 81.00±16.35, animals in groups 0, 25 and 50% HPCS had approximately 10g increase in body weight but those on 100% HPCS had weight loss of -18.00±7.53g as shown in Figure 2.
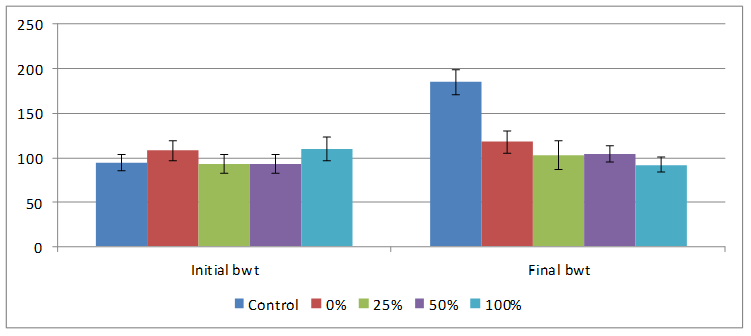
Figure 2. Effect of feeding different percentages of HPCS on body weight
Reduced feed-intake was observed in rats on all experimental diets in this study, this implies that formulated-feed was not as palatable and acceptable to the animals as the regular rat chow (control feed). This reduced acceptability by the rats is supported by our data on feed intake which had decreased tremendously to about 60% by the fourth week and is further evidenced by decrease in body-weights of the animals to control (Figure 2).
Control rats had biggest liver and kidney weights of 3.24±0.25 and 1.03±0.10 respectively Rats fed varying percentages of HPCS had decreased organ weights compared to the control, only those on 50% HPCS diet had liver weight been significantly lower compared to both control and 0% HPCS groups as shown in Figure 3.
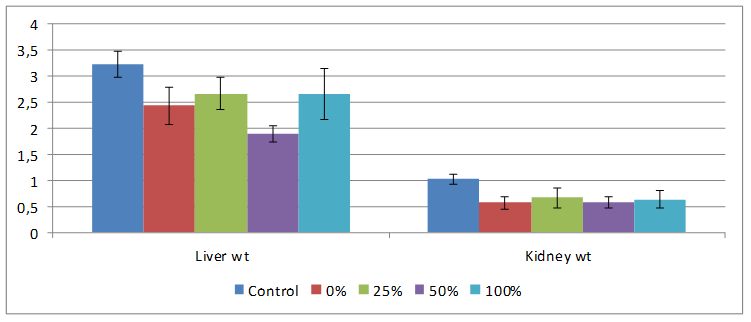
Figure 3. Effect of feeding different percentages of HPCS on organ weights
Rats on HPCS and NCS generally lost weight compared to control because of diet given and this lower body weight might have affected the organ weights as well as shown in Figure 3. Organ-body weight ratio may be used as an index of organ swelling, atrophy, or inflammation [12]. The absence of differences in the liver- and kidney-body weight ratio of animals fed HPCS diet is an indication that HPCS feeding did not produce any swelling or inflammation of the organs. By contrast, the increase in the heart-body weight ratio of the rats maintained on the 50% HPCS diet may imply swelling or hypertrophy of the organ and this might imply that the diet elicits toxic effects.
Effect of feeding different degrees of HPCS on markers of liver toxicity
Markers of liver tissue toxicity were evaluated using alanine amino transferase ALT, aspartate amino transferase AST and alkaline phosphatases ALP are shown on Figure 4. ALT was significantly increased (p<0.05) in the 25, 50 and 100% HPCS fed groups compared to control and NCS fed groups. 0, 50 and 100% HPCS had AST values which were significantly decreased compared to rats fed normal rat chow (control), while 25 and 50% HPCS fed groups had ALP values which were significantly increased relative to normal control rats. Both NCS and HPCS feeding for twenty-eight days caused increase in serum ALT in the liver (Figure 4), elevations in the transaminases levels are considered as the most sensitive markers in the diagnosis of hepatocellular damage and loss of functional integrity of the membrane. Yakubu et al., attributed the increases in serum aminotransferase enzyme activities to their intracellular location in the cytosol, so toxicity affecting the liver with subsequent breakdown in membrane architecture of the cells leads to their spillage into cellular flow where their concentration rises [13]. Leakage of aminotransferases from the liver to the serum thus indicates liver tissue damage. ALP is a ‘‘marker’’ enzyme for the plasma membrane and endoplasmic reticulum, and often it is used to assess the integrity of the plasma membrane [13]. The increase in the serum enzyme levels may be adduced to loss of intact tissue membrane components including ALP from the tissues into the serum [14]. This is an indication that the ordered lipid bilayer of the membrane structure has been damaged or disrupted, leading to the escape of detectable quantity of the enzyme out of the cells of the hepactocytes and nephrons into the extracellular fluid, the serum [14], thus denoting tissue damage.
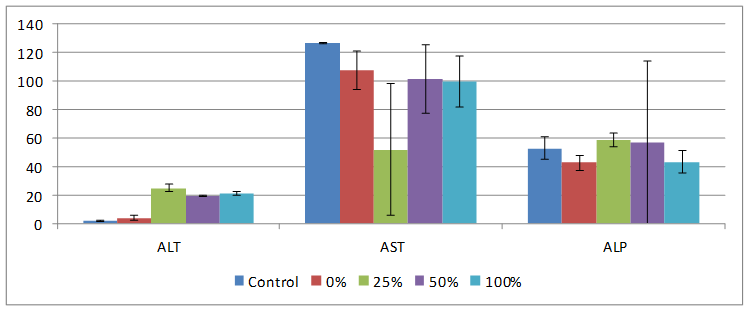
Figure 4. Effect of feeding different percentages of HPCS on serum ALT, AST and ALP
Effect of feeding different degrees of HPCS markers of kidney tissue toxicity
Results obtained for effect of HPCS feeding on creatinine, urea, serum protein and G6PD are shown on Figures 5 and 6. HPCS feeding caused serum creatinine to be significantly elevated (p<0.05) in both 25 and 100% fed groups compared to rats on control and native starch diets. Urea in rats on HPCS decreased compared to those on normal commercial rat feed. Protein concentration in all treatment groups decreased but only in 50% HPCS treated group was the value significantly decreased compared to control. While G6PD increased in all groups on HPCS but only in 25% HPCS rats were the values increased significantly compared to control.
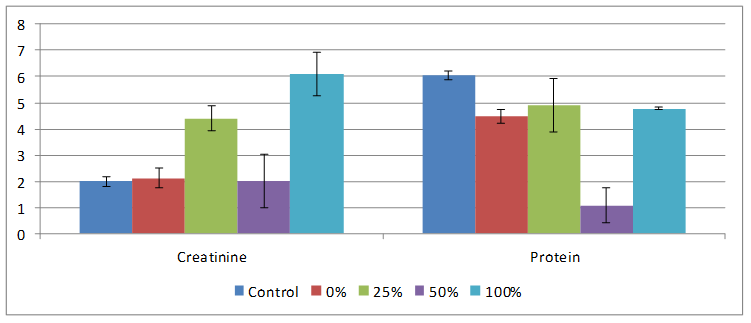
Figure 5. Effect of feeding different percentages of HPCS on serum creatinine and protein
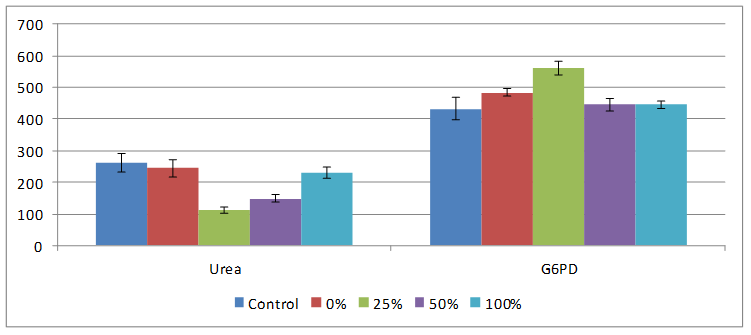
Figure 6. Effect of feeding different percentages of HPCS on serum urea level and G6PD activity
Creatinine, urea, protein and G6PD levels are known predictors of kidney tissue integrity. Elevations in plasma creatinine levels is indicative of under excretion, hence suggesting kidney impairment [15]. In this study, ceatinine significantly increased in rats fed 25% and 100% HPCS starch compared to control, 50% HPCS and NCS, hence test diet did not adversely affect kidney. The decreased levels of serum urea in the rats fed HPCS and NCS diets could be attributed to a decline in liver function as the liver is the primary site for the synthesis of urea. During hepatic injury, blood urea level decreases significantly due to the failure of the liver to convert amino acids and ammonia to urea. G6PD is an enzyme in the pentose phosphate pathway of carbohydrate metabolism and its deficiency results in G6PD haemolytic anaemia. Low levels of G6PD activity, causes a decrease in NADPH and reduced glutathione (GSH) enhance resulting precipitation of deformed haemoglobin, Heinz body formation and red blood cell lysis. In this study NCS and HPCS significantly increased the level of G6PD compared to control. Protein concentrations decreased slightly in rats fed both NCS and HPCS compared to control and altered serum protein is associated with diseased state [16]. Hence, observed results are therefore not indicative of kidney tissue damage in this study.
Effect of feeding different degrees of HPCS on liver and kidney PMF antioxidant in rats
On Table 3 is the data obtained on effect of HPCS feeding on lipid peroxidation and enzymatic antioxidants. Liver MDA values increased compared to control and only in groups on 25, 50 and 100% fed rats were the increase statistically significant (p<0.05) but only 100 HPCS was kidney MDA increased compared to control. Liver SOD was increased in the 0. 25 and 50% HPCS treated groups relative to control while kidney SOD was significantly decreased in all treatment groups compared to control except in rats on native cassava starch. Catalase in the liver increased was significantly by HPCS feeding and in kidney only in the 50% HPCS group was the increase significant.
Table 3. Effect of feeding of HPCS on lipid peroxidation in liver and kidney of rats
|
Organs |
Control |
0% |
25% |
50% |
100% |
|
Liver MDA unit/mg protein |
0.02±0.01 |
0.26±0.02 |
0.75±0.12*# |
0.12±0.04*# |
0.60±0.06*# |
|
Kidney MDA unit/mg protein |
0.62±0.02 |
0.58±0.06 |
0.60±0.12 |
0.44±0.29 |
0.66±0.15 |
|
Liver SOD unit/mg protein |
0.03±0.01 |
0.16±0.01 |
0.15±0.01 |
0.05±0.02 |
0.03±0.02 |
|
Kidney SOD unit/mg protein |
0.12±0.01 |
0.28±0.02 |
0.08±0.07*# |
0.06±0.05*# |
0.04±0.01* |
|
Liver CAT unit/mg protein |
11.42±0.85 |
16.52±3.51* |
17.09±3.06* |
14.48±6.63 |
13.68±0.09* |
|
Kidney CAT unit/mg protein |
12.67±0.04 |
13.46±2.77 |
16.93±3.63 |
14.75±7.49* |
15.74±5.39 |
|
Data are presented as mean ± SEM of six rats in each group: * P<0.05 is significant when compared with control # P<0.05 is significant when compared with rats fed on unmodified cassava starch |
|||||
Lipidperoxidation is an index tissue oxidative damage to membrane lipids [17]. There was a significant increase in MDA levels in the liver of rats fed 25%, 50% and 100% HPCS starch compared NCS and control but not in the kidney, thus implying that there was damage to liver caused by HPCS intake in rats. In order to overcome oxidative stress, cells are equipped with antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT), SOD catalysis the reaction superoxide anion radical to hydrogen peroxide and catalase reduces it to water [17]. The increased activities of the antioxidants enzyme could be as a response to mop up accumulated reactive oxygen or increased lipid peroxidation species caused by NCS and HPCS feeding which could have deleterious effect on membrane integrity and function. CAT and SOD activities were increased in rats NCS and HPCS in the liver but not in the kidney, thus the increase implies an activation in the antioxidative enzymes in response increased oxidative stress elicited by dietary intake compared to control.
Effect of feeding different degrees of HPCS on micronutrient and cyanide levels in rats
Result obtained on effect of feeding rats with diet compounded with varying percentages of HPCS on plasma levels of zinc, selenium and cyanide are shown on Table 4. HPCS feeding did not affect cyanide levels in rats as there were no significant differences. However, Zn and Se were significantly decreased in rats fed 50% HPCS compared to control.
Table 4. Effect of feeding HPCS on plasma levels of zinc, selenium and cyanide
|
|
CONTROL |
0% |
25% |
50% |
100% |
|
Zinc |
22.624±1.197 |
23.573±3.655 |
23.316±2.824 |
20.046±1.500* |
23.000±0.779 |
|
Selenium |
0.0357±0.0031 |
0.034±0.0072 |
0.0357±0.00569 |
0.0283±0.00153* |
0.0347±0.055 |
|
Cyanide |
0.0123±0.00153 |
0.0107±0.0025 |
0.0147±0.0057 |
0.012±0.0200 |
0.011±0.002 |
|
Data are presented as mean ± SEM of six rats in each group * P<0.05 is significant when compared with control # P<0.05 is significant when compared with rats fed on unmodified cassava starch |
|||||
Hydroxypropylation did not cause any significant difference the NCS and HPCS feeding to rats compared to control and this is very important as it indicates that there was no cyanide intoxication, that cassava starch used in this study was safe for animal consumption and had adequate content of essential trace elements such as selenium and zinc.
Effect of feeding different degrees of HPCS on liver tissue histopathology
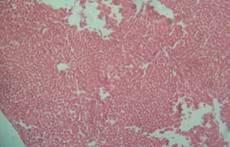
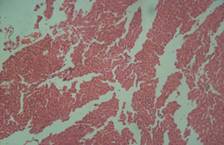
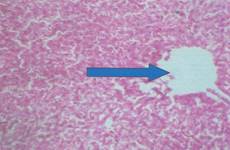
There was no significant lesion in the liver of rats on normal rat chow, native starch as well as 25 and 50% HPCS, however animals on 100% HPCS for four weeks had centrilobular fatty degeneration and congested veins in the tissue histograph (Figure 7a, b, c, d and e). Kidney tissues had normal tissue histology for all treatment groups and as such have not been included for simplicity of data.
|
(a) control showing no lesions |
(b) native cassava starch showing no lesions |
(c) 25% HPCS showing no lesions |
|
|
(d) 50% HPCS showing no lesions |
(e) 100% HPCS arrow showing centrilobular fatty degeneration and congested veins |
||
Figure 7. Liver tissue histology
Histopathology examination showed normal tissue morphology in the kidney, heart and in the liver only the rats on 100% HPCS had centrilobular fatty degeneration and congested veins in the tissue histograph Histopathology phoptomimrogaph for kidney and heart tissue were not included for simplicity of data, however normal kidney morphology obtained was in contrast with serum creatinine result (Figure 7 a-e) which indicated tissue impairment and there is need for caution in interpreting such results. Shahjahan et al., had earlier observed that if exposure to a supposed toxicant is low and possibly of a short duration the kidneys could repair the damaged cells and function normally again [18].
Conclusion
Our results indicate that HPCS intake for four weeks, especially when used as total replacement of carbohydrate source in the diet may have possible nephrotoxic and hepatotoxic effects at different levels of modification, it may lead to increase of reactive oxygen species, hence the need for caution.
Also, caution should be exercised in the use of either NCS or HPCS in feed formulation for rats for continual ingestion for four weeks based on both fee intake and bodyweight changes in this study despite the fact that there were no significant differences in cyanide levels.
Observed differences might be due mainly to diminished protein content of cassava starch compared to the grains. Thus, feed formulation from NCS might require fortification for good growth performance.
Acknowledgements
We appreciate Industrial Chemistry Laboratory for the donation of HPCS and Mr Eric Sabo for technical assistance.
References
1. Mason W.R., Starch Chemistry and technology, Academic Press, London, UK, 2009.
2. Wolf B.W., Wolever T.M.S., Bolognesi C., Zinker B.A., Garleb K.A., Firkins J.F., Glycemic response to a food starch esterified by 1-octenyl succinic anhydride in humans, J. Agr. Food Chem. 2001, 49, p. 2674-2678.
3. Wolf B.W., Bauer L.L., Fahey Jr G.C., Effects of chemical modification on in vitro rate and extent of food starch digestion: an attempt to discover a slowly digested starch. J. Agric. Food Chem., 1999, 47, p. 4178–4183.
4. BeMiller J.N., Carbohydrate chemistry for food scientist. St Paul. MN: AACC International, 2007, p. 173-223.
5. Ernesto M., Cardoso A.P., Nicala D., Mirione E., Massaza F., Cliff J., Haque M.R., Bradbury J.H., Persistent konzo and cyanogens toxicity from cassava in northern Mozambique, Acta Tropica, 2002, 82, p. 357-362.
6. Akintonwa A., Tunwashe O.L., Fatal cyanide poisoning from cassava-based meal, Human and Experimental Toxicology, 1992, 11, p. 47-49.
7. AOAC, Official Methods of Analysis of the Association of Official Analytical Chemists, 15th Edition, Washington, DC. 1990.
8. Nwozo S.O., Orojobi F., Adaramoye O.A., Hypolipidemic and antioxidant potentials of Xylopia aethiopica seed extract in hypercholesterolaemic rats, Journal of Medicinal Foods, 2011, 14 (1/2), p. 114-119.
9. Moore G.R.P., Canto L.R., Soldi V., Amante E.R., Cassava and corn starch in maltodextrin production, Quı´mica Nova, 2005, 28, p. 596–600.
10. Singh J., Kaur L., McCarthy O.J., Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications-A review, Food Hydrocology, 2007, 21, p. 1-22.
11. Abbas K.A., Khalil K.S., Hussin A.S.M., Modified starches and their usages in selected food products: A review study, Journal of Agric. Sci., 2010, 2(2), p. 90-100.
12. Ramakrishnan G., Augustine T.A., Jagan S., Vinodhkumar R., Devaki T., Effect of silymarin on N-nitrosodiethylamine induced hepatocarcinogenesis in rats, Experimental Oncology, 2007, 29, p. 39-44.
13. Yakubu M.T., Bilbis L.S., Lawal M., Akanji M.A., Effect of repeated administration of sildenafil citrate on selected enzyme activities of liver and kidney of male albino rats, Nigerian Journal of Pure and Applied Sciences 2003, 18, p. 1395–1400.
14. Ghorbe F., Boujelbene M., Makni-Ayadi F., Guermazi F., Kammoun A., Murat J., Croute F., Soleiihavoup J.P., Feki A.E., Effect of chronic lead exposure on kidney function in male and female rats: determination of a lead exposure biomarker, Physiology and Biochem, 2001, 5(109), p. 457-463.
15. National Academy of Clinical Biochemistry (NACB), Laboratory Medicine Practice Guidelines, Edited by Dufour R.D., 2000.
16. Veena C.K., Josephine A., Preetha S.P., Varalashmi P., Effect of sulphated ysaccharides on erythrocyte changes due to oxidative stress in experimental hyperoxularia, Hum Exp Toxicol, 2007, 26, p. 923-932.
17. Vásquez-Garzón V.R., Arellanes-Robledo J., García-Román R., Aparicio-Rautista D.I., Villa-Treviño S., Inhibition of reactive oxygen species and pre-neoplastic lesions by quercetin through an antioxidant defense mechanism, Free Radical Research, 2009, 43, p. 128–137.
18. Sharhjahan M., Sabitha K. E., Mallika J., Shyamala-Devi C. S., Effect of Solanum trilobatum against carbon tetrachloride induced hepatic damage in Albino rats, Indian J. Med. Res., 2004, 120, p. 194-198.