Engineering, Environment
Under-deposit corrosion behaviour of API 5L-X65 steel in CO2 environment containing Sida Acuta extract
Sunday ARIBO1, 2, Ekiomoadona Melody INEGBEDION1, Sunday Joseph OLUSEGUN1,4 *, Oladeji Oluremi IGE2,3, Adekunle Sulaimon OGUNBADEJO1, Peter Apata OLUBAMBI2
1Department of Metallurgical and Materials Engineering, Federal University of Technology, Akure, Nigeria
2Centre for Nanoengineering and Tribocorosion, School of Mining, Metallurgy and Chemical Engineering, University of Johannesburg, Johannesburg, South Africa
3Department of Materials Science and Engineering, Obafemi Awolowo University, Ile-Ife, Nigeria
4Universidade Federal de Minas Gerais, Departamento de Química, Laboratório de Materiais Nanoestruturados, Belo Horizonte, Brazil
Email(s): aribosunny@yahoo.com, ige4usa@yahoo.com, adekunleogunbadejo@gmail.com,
polubambi@gmail.com, ekipretty@yahoo.com, *arewasegun@gmail
*Corresponding author, phone: +5531975672191
Received: October 31, 2017/ Accepted: December 27, 2017 / Published: December 30, 2017
Abstract
Effects of sida acuta extract on the corrosion behaviour of API 5L-X65 steel under sand deposit in CO2 saturated oilfield brine has been studied. Tafel extrapolation method has been employed to determine the corrosion rate of the alloy under sand deposit with and without sida acuta extract. Atomic force microscopy was employed to study the surface roughness of the steel. Phytochemical analysis and Fourier transformed infrared (FTIR) spectroscopy was used to identify the functional groups present in the extract. The presence of sand retarded the inhibitor efficiency at lower dosage but better corrosion inhibition was obtained at higher inhibitor dosage.
Keywords
Sida acuta; Sand deposit; Corrosion; FTIR, AFM, Adsorption isotherm
Introduction
One form of corrosion that is prevailing in the oil and gas industries is the CO2 corrosion; this has been a great concern in the industries because of its aftermath effects [1]. The enormous consequences of CO2 corrosion in oil and gas industries have resulted to leakages of crude oil, environmental pollution, shutdown of plants, fire accidents and lots more [2]. The association of CO2 in the aqueous phase results in the formation of carbonic acid which further dissociates to form bicarbonate and carbonate ions; this leads to the formation of corrosion product (FeCO3), which could form a protective or non-protective film on the metal surface when precipitated. However this has been affirmed to be a function of the environmental conditions [3,4]. One method used to mitigate CO2 corrosion is the use of inhibitors [5,6]. The use of inhibitors has been well documented as an effective method of protecting metallic materials from corrosion [7]. This is because of their abilities to impede the anodic or cathodic reaction by blocking the active sites on the steel surface [8].
Both organic and inorganic inhibitors are effective in the oilfield. However, the toxic effects of synthetic corrosion inhibitors have led to the search for naturally occurring substances which are not only readily available but are also environmentally friendly [9,10]. Green inhibitors obtained from plants are biodegradable and do not contain heavy metals or other toxic compounds; they are eco-friendly [11,12]. In view of these, plant extracts can now serve as corrosion inhibitors because they contain wide variety of organic compounds having polar atoms such as oxygen, nitrogen, phosphorus and sulphur [13,14]. It is also of note that solids like sands and scales could be found at the bottom of pipelines forming bed of solids. Such solid deposits have been found to be source of many problems such as loss of inhibitor; galvanic and other forms of localised corrosion [15].
This research is thus an attempt to study the inhibition behaviour of a green inhibitor under sand bed in CO2-saturated oilfield brine.
Material and method
Mild steel API 5L-X65 with composition C = 0.154%, Si = 0.45%, Mn = 1.357%, P = 0.023%, S = 0.014%, Cr = 0.061, Cu = 0.001, Ni = 0.022, and the remaining % of Fe was used for this research. Extract from sida acuta plant, silica sand, NaCl salt, CO2 gas, concentrated ethanol, and distilled water were also used.
Extraction and characterization sida acuta extract
Extraction process was carried in line with the method reported by Alaneme et al., [16]. After the extraction, specific grams of the extract were weighed (0.1 0.2. 0.3 and 0.4) and dissolved in one litre of 3.5 wt. % NaCl solution for the corrosion study. Phytochemical analysis of the extracted sida acuta was carried out according to the standard methods as reported by Olusegun et al., [17]. The presence of phenol, alkaloid, tannin, saponnin and flavonoid were identified in the extract. Fourier transformed infrared (FTIR) spectra measurement of the extract was carried out on Perkin-Elmer spectrophotometer using the KBr pellets.
Potentiodynamic polarization measurement
The working electrodes were prepared by attaching an insulated copper wire to one face of the sample using an aluminium conducting tape, and cold mounted in resin. The sample was then immersed into the prepared environment for electrochemical measurements. Tafel polarisation method was employed for the electrochemical measurements by polarizing the surface of the sample from a potential of -250 mV below OCP to a potential of +250 mV above OCP at a scan rate of 0.25 mV/s. Meanwhile before each experiment, the open circuit potential was allowed to settle for 1 hour. Experimental methods for the under deposit corrosion was carried out as reported by Aribo [15]. Corrosion experiments were conducted in a simulated oilfield brine solution with and without inhibitor under stagnant CO2 saturated condition at 250C. Formation of the oilfield brine was done by purging the solution of 3.5 wt. % NaCl with CO2 gas for 2 hours before the experiment. The solution was also continually purged with CO2 throughout the duration of the experiment.
The pH of the environments is shown in Table 1.
Table 1. pH of the solution
|
Experiment |
pH before purging with CO2 |
pH after purging with CO2 |
pH after experiment |
|
Blank + sand |
6.4 |
4.0 |
4.3 |
|
Blank+sand+100ppm inhibitor |
6.5 |
4.4 |
4.4 |
|
Blank+sand+200ppm inhibitor |
6.2 |
4.6 |
4.5 |
|
Blank+sand+300ppm inhibitor |
6.2 |
4.5 |
4.5 |
|
Blank+sand+400ppm inhibitor |
6.2 |
4.5 |
4.5 |
The inhibition efficiency of the extract at various concentrations was calculated using Eq. (1):
IE % = (Iocorr - Icorr/ Iocorr) 100 (1)
Where: I0corr
and Icorr - are the corrosion current densities of the
blank and extract solution respectively; ![]() - is
the inhibition efficiency.
- is
the inhibition efficiency.
After evaluating several isotherms it was observed that the data fits well with Freundlich isotherm. The isotherm is expressed by the equation in Eq. (2):
![]() (2)
(2)
Where: C - is
extract concentration; Kads - is Freundlich adsorption equilibrium
constant; ![]() -
is the surface coverage and 1/n heterogeneity factor.
-
is the surface coverage and 1/n heterogeneity factor.
Figure 1 shows the scheme of the methodology.
Figure. 1 Scheme of the methodology
Results and discussion
FTIR analysis
Figure 2 shows the FTIR spectrum of the extract at different bands. The broad peaks at 3332 cm-1 is due to OH stretching vibration, 2934 cm-1 band correspond to –CH, the sharp peak at 1630 cm-1 is attributed to primary amine (-NH), the peak at 1402 cm-1 is due to C=C, while C-O is identified at 1068 cm-1 peak.
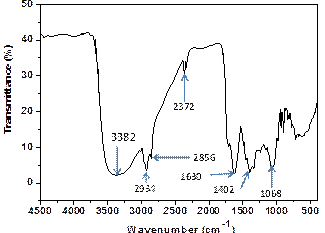
Figure 2. IR spectra of sida acuta
Studies have shown that compounds that contain elements like N, O, S and P in their conjugated system have proven to prevent the aggressive dissolution of metallic materials in corrosive medium [18,19]. The mechanism of the corrosion inhibition of these compounds have been suggested to be as a result of their adsorption on the active sites of metal surface, these process would lead to the blockage of the sites resulting in a decrease in the rate of corrosion [19,20].
Electrochemical measurement
Inhibitor dosage of 100, 200, 300 and 400 ppm were tested. The mild steel is significantly inhibited against corrosion at higher inhibition dosages of 300 ppm and 400 ppm. There is also a gradual decrease of the corrosion rate with addition of 100 ppm and 200 ppm inhibitor dosage.
Also, the addition of sida acuta results in an increase of Ecorr towards positive direction suggesting that the steel shows a reduced corrosion rate. The result of electrochemical measure is shown in Figure 3.
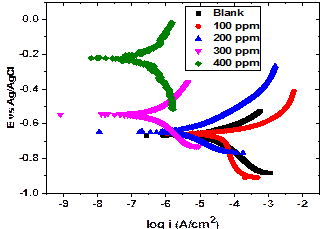
Figure 3. Tafel plots for the mild steel under sand deposit with and without sida acuta at 25oC
Although there is no significant reduction of corrosion rate at 100 ppm, however, there is suppression of cathodic reaction in the inhibited solution. This is an indication of a reduced corrosion reaction. Also, there is slight negative shift in corrosion potential (Ecorr) of steel without inhibitor compared to steel with inhibitor. This indicates the nobility of the inhibited steel and reduced corrosion behaviour. A shift of 130 mV and 460 mV anodically in the Ecorr at 300 and 400 ppm inhibition respectively indicates that the inhibitor is anodic. This is because it has been reported that a shift of more than 85 mV in either anodic or cathodic direction can establish if the inhibitor is anodic or cathodic [21, 22].
There seems to be little or no effects of the increase dosage of the inhibitor on the pH of the solution before and after purging (Table 1).
Though purging of the aerated solution with CO2 reduced the pH generally as expected, increasing the inhibition dosage seems to have little or no effect. Corrosion parameter and inhibition efficiency are listed in Table 2.
Table 2. Corrosion parameter and inhibition efficiency
|
Parameter |
Current density (µA/cm2) |
Corrosion rate (mm/yr) |
Corrosion potential (V) |
Inhibition efficiency (%) |
|
Blank + sand |
100 |
1.17 |
-0.680 |
|
|
Sand + 100 ppm |
75.5 |
0.878 |
-0.680 |
25 |
|
Sand + 200 ppm |
37.4 |
0.411 |
-0.648 |
63 |
|
Sand + 300 ppm |
18.7 |
0.219 |
-0.550 |
81 |
|
Sand + 400 ppm |
9.6 |
0.1122 |
-0.223 |
91 |
Inhibition efficiency
Inhibition efficiency of 81 and 91 % were recorded at dosages of 300 and 400 ppm respectively as itemised in Table 2. The lower efficiencies experienced at 100 and 200 ppm are not unexpected as such have been earlier reported for Jathropha Curcas inhibitor at the same temperature under the same environmental conditions [9]. The beauty of the results is that inhibition efficiency increased as the concentration of dosage increased. Increase in inhibition efficiency as concentration increases has been reported to be due to the increase in the number of constituent molecules of inhibitor adsorbed on the metal surface at higher concentrations [23].
Adsorption isotherm
The data were well fitted into Freundlich isotherm as shown in Figure 4. The implication of this is that sida acuta extract adsorption is a non-ideal and reversible; and that it is not restricted to monolayer formation [24].
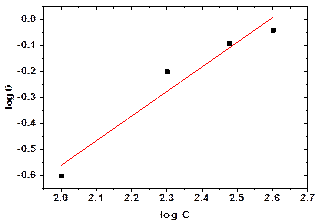
Figure 4. Freundlich isotherm of sida acuta at 298K in CO2 environment under sand deposit
AFM images
The effectiveness of the extract was shown by AFM analyses (Figure 5). This gave insight about the surface roughness of each of the material with and without inhibitor. The surface of the steel after immersion in the test solution without inhibitor shows some characteristics of coarse and rough surface. This is due to anodic dissolution of the steel resulting into pitting and general corrosion. The presence of inhibitor hindered the damage that could be caused by the corrosive species; this was made possible due the adsorption of the active constituent (like tannin, saponnin and flavonoid) of the extract on the surface of the mild steel. These micrographs showed that the extract effectively reduce the damage that could be caused by the aggressive solution.
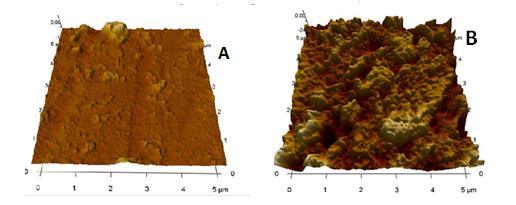
Figure 5. AFM 3D images of API 5L-X65 Steel after immersion with inhibitor (A); after immersion without inhibitor (B)
Conclusions
The inhibitor effectiveness of sida acuta extract has been studied by Tafel extrapolation and AFM analyses. The result obtained showed that the extract could serve as effective corrosion inhibitor for mild steel protection in CO2 under deposit environment. The efficiency of the extract increased with increase in concentration and the adsorption isotherm is consistent with Freundlich isotherm. The presence of functional groups in the extract as identified by FTIR analysis was responsible for the retardation of the corrosion process.
Acknowledgment
The authors would like to thank Universidade Federal de Minas Gerais-Microscopy Center and Professor Nelcy D. S. Mohallem for their support.
References
1. Zhu S.D., Fu A.Q., Miao J., Yin Z.F., Zhou GS, Wei JF. Corrosion of N80 carbon steel in oil field formation water containing CO2 in the absence and presence of acetic acid, Corros Sci., 2011, 53 (10), p. 3156–65.
2. Zhang G, Lu M, Chai C, Wu Y. Effect of HCO3- concentration on CO2 corrosion in oil and gas fields, J. Univ. Sci. Technol. Beijing Miner Metall Mater (Eng Ed.), 2006, 13 (1) p. 44–9.
3. Ag B.D., Fundamental aspects of CO2 metal loss corrosion-part 1: mechanism, Corrosion, 2006, (6111), 1–18.
4. Forero A.B., Bott I.S., Núñez M.M.G., Analysis of the corrosion scales formed on API 5L X70 and X80 steel pipe in the presence of CO2, Mater Res. 2014, 17 (2), p. 461–71.
5. Zhao J., Chen G., The synergistic inhibition effect of oleic-based imidazoline and sodium benzoate on mild steel corrosion in a CO2-saturated brine solution, Electrochim Acta, 2012, 69, p. 247–55.
6. Ghareba S., Omanovic S., The effect of electrolyte flow on the performance of 12-aminododecanoic acid as a carbon steel corrosion inhibitor in CO2-saturated hydrochloric acid, Corros Sci., 2011, 53 (11), p. 3805–12.
7. Tosun A, Ergun M., Protection of corrosion of carbon steel by inhibitors in chloride containing solutions, Corrosion, 2006, 19 (3), p. 149–54.
8. Popoola L., Grema A., Latinwo G., Gutti B., Balogun A., Corrosion problems during oil and gas production and its mitigation, Int. J. Ind., 2013, 4 (1), p. 35.
9. Aribo S., Olusegun S.J., Ibhadiyi L.J., Oyetunji A., Folorunso D.O., Green inhibitors for corrosion protection in acidizing oilfield environment, J. Assoc. Arab Univ. Basic Appl. Sci., 2017, 24, p. 34-38.
10. Olasehinde E.F., Olusegun S.J. , Adesina A.S., Omogbehin S.A., Momoh-Yahayah H. Inhibitory action of nicotiana tabacum extracts on the corrosion of mild steel in HCl: Adsorption and thermodynamics study, Nat. Sci., 2013, 11 (1), p. 83–90.
11. Eddy N.O., Odoemelam S.A., Odiongenyi A.O., Joint effect of halides and ethanol extract of Lasianthera africana on inhibition of corrosion of mild steel in H2SO4, J. Appl. Electrochem. 2009, 39 (6), p. 849–57.
12. Beenakumari K.S., Inhibitory effects of Murraya koenigii (curry leaf) leaf extract on the corrosion of mild steel in 1 M HCl inhibitory effects of Murraya koenigii (curry leaf) leaf extract on the corrosion of mild steel in 1 M HCl, Green Chem. Lett. Rev. 2011, 4 (2), p. 117–20.
13. Fouda A.S., Shalabi K., Idress A.A., Thymus vulgarise extract as nontoxic corrosion inhibitor for copper and α-brass in 1 M HNO3 solutions, Int. J. Electrochem. Sci. 2014, 9 (9), p. 5126–54.
14. Finsgar M., Jackson J., Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review, Corros. Sci., 2014, 86, p. 17–41.
15. Aribo S., Sanumi O.J., Olusegun S.J., Ogunbadejo A.S., Ige O.O., Jatropha Curcas as a corrosion inhibitor for API 5L-X65 steel under sand deposit in CO2 and aerated oilfield environments, African Corros J. 2016, 2 (2), p. 17–27.
16. Alaneme K.K., Olusegun S.J., Alo A.W., Corrosion inhibitory properties of elephant grass (Pennisetum purpureum) extract: Effect on mild steel corrosion in 1M HCl solution, Alexandria Eng. J., 2016, 55 (2), p. 1069–76.
17. Olusegun S.J., Oluwasina O.O., Alaneme K.K., Olubambi P.A., Corrosion inhibition of mild steel in acidic solution by cow dung extract as an eco-friendly inhibitor, J. Mater. Environ. Sci. 2016, 7 (4), p. 1086–97.
18. Feng L., Yang H., Wang F., Experimental and theoretical studies for corrosion inhibition of carbon steel by imidazoline derivative in 5% NaCl saturated Ca(OH)2 solution, Electrochim. Acta, 2011, 58 (1), p. 427–36.
19. Vinutha M.R., Venkatesha T.V., Review on mechanistic action of inhibitors on steel corrosion in acidic media, Port Electrochim. Acta, 2016, 34 (3), p. 157–84.
20. Maksoud S.A.E., The effect of organic compounds on the electrochemical behaviour of steel in acidic media. A review, Intl. J. Electrochem Sci., 2008, 3, p. 528–55.
21. Li WH, He Q., Zhang S.T., Pei C.L., Hou B.R., Some new triazole derivatives as inhibitors for mild steel corrosion in acidic medium, J. Appl. Electrochem., 2008, 38 (3), p. 289–95.
22. Ferreira E.S., Giacomelli C., Giacomelli F.C., Spinelli A., Evaluation of the inhibitor effect of L-ascorbic acid on the corrosion of mild steel, Mater. Chem. Phys. 2004, 83 (1), p. 129–34.
23. Bea R., Basu B.B.J., Green inhibitors for corrosion protection of metals and alloys: An overview, Int. J. Corros, 2012.
24. Ige O.O., Barker R., Hu X., Umoru L.E., Neville A., Assessing the influence of shear stress and particle impingement on inhibitor efficiency through the application of in-situ electrochemistry in a CO2-saturated environment, Wear, 2013, 304 (1–2), p. 49–59.