Engineering, Environment
Comparing the antioxidant extract of the quince's peel with hexane and ethyl acetate solvents for oxidation of crude soybean oil prevention
Abolfazl Deh ABADI1, Vahid HAKIMZADEH*2, Hasan RASHIDI3
1, 2 Department of Food science and technology, Quchan Branch, Islamic Azad University, Quchan, Iran
3 Food Department, Khorasan Razavi Agriculture and Natural Resources Research and education center, AREEO, Mashhad, Iran
E-mail(s): 1abolfazldehabadi@yahoo.com; *2v.hakimzadeh@yahoo.com; 3ha_rashidi@yahoo.com
* Corresponding author, phone: +989155124802 fax: +983433370557
Received: September 05, 2017 / Accepted: December 27, 2017 / Published: June 30, 2018
Abstract
Nowadays, due to the importance of waste management in the food industry, reuse of waste processing and increase their added value , the extraction of functional ingredients such as natural antioxidants is growing. In this study, the effect of antioxidant quince peel in oil oxidation stability by the peroxide and thiobarbituric acid tests was investigated using non-polar solvent n- hexane and ethyl acetate as polar solvent in various concentrations of zero (control), 300, 600 and 900 ppm in crude soybean oil. Also, the effect of 100 ppm of BHT and mixed the BHT with the extracts by same ratio were compared with the effect of antioxidant quince's peel on the stability of crude soybean oil. The results showed that with increasing the concentration of the quince peel extract, oxidation stability improved. Also the concentrations higher than 600 ppm were more effective and almost similar to effect of 100 ppm BHT. The synergistic effect of ethyl acetate extract was obvious using the synthetic antioxidant BHT on oxidative stability of crude soybean oil, while this effect was not noticeable for the extracts from hexane solvent. Overall, ethyl acetate extracts have shown to be more effective due to the same polarity with phenolic compounds in oxidative stability of crude soybean oil.
Keywords
BHT; Ethyl acetate; N-hexane; Quince; Soybean oil
Introduction
Currently, because of the importance of waste management in the food industry and the reuse of waste processing, their added value and the extraction of functional ingredients for other sectors of the food industry is increasing [1]. Every day, large quantities of fruits peel and other edible parts during the processing or their direct consumption is become waste and therefore thrown away. Food wastes are considered not only as animal feed or fertilizers are used as a valuable resource, but also accessible and cheap to extract as valuable compounds. These materials can be beneficial to obtain compounds such as antioxidants, enzymes, antimicrobial compounds, nutrients and etc. [2]. Carotenoids and phenolic compounds are effective for creating colour and flavour in fruits and vegetables and they also contain antioxidant properties which mostly find in fruits and vegetables peels. Over the past two decades, most of researchers have focused on the discovery of new natural antioxidants from plant resources because using synthetic antioxidant in food industry has high risks for human health and therefore is being limited [3]. Carotenoids are also commonly acted as secondary antioxidants in the absence of singlet oxygen by trapping free radicals to prevent oxidation. Carotenoids such as Lutein are also proven to have some health benefits [4]. Oil oxidation changes its organoleptic characteristics and nutritional value in addition to reducing maintaining oil life. Currently, synthetic antioxidants such as BHA, BHT and TBHQ is used to prevent oxidation of raw oils, however since synthetic antioxidants have undesirable effects such as cancer in the human body, they are gradually being removed from the list are consumer antioxidants. Hence, the production of natural antioxidants and investigating it’s the effect of preserving it on as a substitute for oil is essential [5].
In this regard, the quince's peel can be considered as one of the food industry wastes. Quince has a fluffy peel and almost astringent flavour. It is rich in vitamins A and B, minerals calcium and tannins. Anti-cancer properties of the quince are due to the presence of antioxidant compounds. The total amount of phenolic compounds in quince has been reported as 119 mg per kg [6]. Several studies on the effect of fruit peels and vegetable extracts like orange, green tea, potato as natural antioxidants on stability of variety of raw oil have been done.
The aim of this research is investigation of antioxidant potential of quince peel extract in crude soybean oil prevention. Also, the effectiveness of two different solvent in terms of their polarity on the extract antioxidant strength was evaluated.
Materials and methods
In this study, the quince’s peel antioxidant extracts was obtained using n-Hexane as non-polar solvent and Ethyl acetate as polar solvent. The best extract from these two solvents in terms of antioxidant power, was examined as a natural antioxidant, both separately and mixed with synthetic antioxidant, for prevention of oxidation of crude soybean oil.
A summary of the research is shown in the diagram in Figure 1.
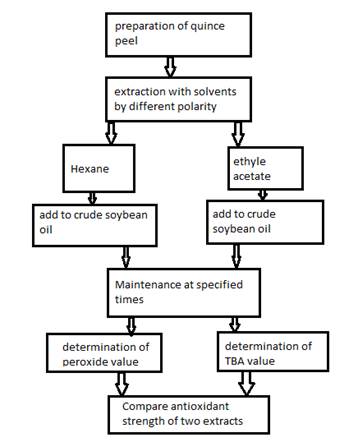
Figure 1. The working algorithm of research steps
Preparation of extract
First, the quince’s peel was dried in a vacuum oven at 40°C for 14 hours at a pressure of 70 mbar. Then it’s powdered was obtained using a laboratory-scale mills and was kept in a dry place without any moisture. Then 10 grams weighed powder transferred to a 500 ml Erlenmeyer flasks follow by adding 300 ml of solvent to (1:30 ratio) in a shaking incubator at 50°C (according to boiling point of solvents) for 12 hours to be extracted [7]. The extract was then filtered using a filter paper and remain solvents in extracts was removed in a rotary evaporator under vacuum at 40°C at a speed of 70 rpm and under reduced pressure until its weight become constant. Then the extracts were transferred into the bowl and covering with aluminium and was placed in dark surroundings to avoid undesirable changes in antioxidant power [8-10].
Preparation of crude soybean oil samples
Odourless and antioxidant-free crude oil soybean (made from plant Tabarrok Co.-Mashhad-Iran) was weighted in the specified amounts and poured in Erlenmeyer flask. Follow by adding each of extracts in zero concentrations (as a Control sample), 300, 600 and 900 ppm separately. Because of the polarity difference between the compounds in the extract of soybean oil, it was likely to have low solubility, so 0.02 % of sorbitol esters was used as emulsifiers in order to obtain a homogenous oil and extract [11]. Also in another flask, 100 ppm of the synthetic antioxidant BHT was added. In addition to that, 100 ppm of extract mixed (both polar and non-polar) with 100 ppm of antioxidant BHT was added to the crude oil to investigate the synergistic effect of them. After this step, samples were incubated at 60°C in order to accelerate the oxidation and the results were compared at days 4, 8, 16 and 24 index, peroxide and TBA index samples.
Peroxide and thiobarbituric acid test
To investigate the stability of crude oil in the presence of different concentrations of extracts of quince’s peel, synthetic antioxidant and their mixed forms, primary oxidation compound amount examined by peroxide test and secondary oxidation compounds were measured by thiobarbituric acid test [12]. The peroxide index is mile equivalent peroxide in 1000 gr oil or fat. Peroxides or mono-hydroxides are the primary oxidation products that are produced by reaction between oxidation of oil or fat due to the reaction between oxygen and free radicals of fatty acids. But, thiobarbituric acid test indicates the reaction of secondary oxidation combinations and, in particular, malondialdehyde with this acid at 523 nm [11].
Statistical analysis
In this study, the concentration of quince peel extracts and time of crude oil storage as independent parameter investigated on the rate of oil oxidation by determination the Peroxide and TBA indexes. The mean comparison of peroxide and TBA Index in different of concentration during the time was performed by SPSS software using Duncan method and the charts were obtained and plotted by excel software.in Duncan method, the non-homogeneous letters indicate a significant difference and the matching letters indicate that there is no significant difference between the data.
Results and discussion
Ethyl acetate extract. Peroxide value
As it is illustrated in Figure 2, the results showed that the presence of ethyl acetate extract of the quince's peel prevent the progression of oxidation in crude soybean oil. However, the low concentrations of the extract were not as much effective in preventing oxidation. In contrast, higher concentrations of extract were shown to be effective in crude soybean oil corruption preventing, similar to synthetic antioxidants. Synergistic effects of the extract in the presence of antioxidant BHT was obvious in this study.
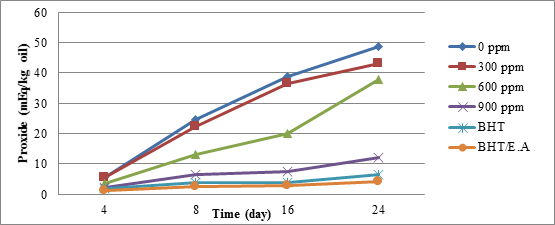
Figure 2. Soybean oil peroxide value changes over time in various amounts of the quinces peel extracts with ethyl acetate
Probably, the low concentrations of the extract did not have the ability to prevent oxidation of the oil at longer times. In fact, it can be said that an appropriate concentration of phenolic compounds is necessary to prevent oxidation, which is not provided in low amounts of quince peel extract. Because of the role of phenolic compounds is more effective than other antioxidants [13]. Obtained results by a study which was an investigation on the effect of Ramos potato peel extract on the stability of crude soybean oil showed that concentrations above 800 ppm of extract was more effective than the effect of antioxidants BHA on the soybean oil [14]. Moreover, in similar research, concentration of 800 ppm of persimmon peel extract in the stability of sunflower oil over the 60 days was effective [5]. Also was shown that green tea leaf extract with antioxidants BHA has a synergistic effect on the stability of synthetic oil compared to the other extracts [11].
Thiobarbituric acid index
Thiobarbituric acid test represents secondary oxidation compounds, particularly malondialdehyde which result in intense flavour variations on eatable oils. As it can be observed in Figure 3, concentrations of malondialdehyde and therefore the intensity of light absorption on the longer days and at lower extracts concentrations increased while this amount was lower in the presence of synthetic antioxidants and high concentration of the quince’s extract and remain stable during the 24 days. Amounts above 600 ppm of both ethyl acetate extract of quince’s peel and mixed extract and synthetic antioxidant, had a similar effect by a narrow border.
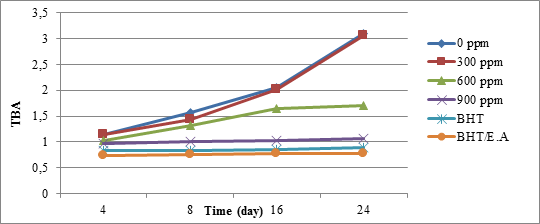
Figure 3. Thiobarbituric acid soybean oil index changes over time in various amounts of the quinces peel extracts with ethyl acetate
Increasing the oxidation velocity in longer days is associated with accelerating the conversion of the primary oxidation compounds to aldehydes.
Table 1 and 2 depict variations in peroxide value and thiobarbituric acid ethyl acetate at various treatments through the time.
Table 1. Compares the average peroxide value during the days and various concentrations of ethyl acetate extract of the quince peel (5%)
|
Extracts average |
24 days |
16 days |
8 days |
4 days |
Treatments |
|
29.45 a |
48.6 |
39 |
24.6 |
5.6 |
0 |
|
26.82 b |
43.2 |
36.5 |
22.2 |
5.4 |
EA-300 |
|
18.65 c |
38 |
20 |
13.2 |
3.4 |
EA-600 |
|
7.05 d |
12.1 |
7.5 |
6.5 |
2.1 |
EA-900 |
|
4.07 e |
6.5 |
4 |
3.9 |
1.9 |
BHT-100 |
|
2.67 f |
4.1 |
3 |
2.5 |
1.1 |
BHT / EA |
|
|
25.42d |
18.33c |
12.15b |
3.21a |
Days average |
Table 2. Compares the average index thiobarbitoric during days and various concentrations of ethyl acetate extract of the quince's peel (5%)
|
Extracts average |
24 days |
16 days |
8 days |
4 days |
Treatments |
|
1.97 a |
3.1 |
2.06 |
1.56 |
1.15 |
0 |
|
1.91 a |
3.05 |
2.01 |
1.43 |
1.15 |
EA-300 |
|
1.4 b |
1.7 |
1.65 |
1.32 |
1.02 |
EA-600 |
|
1.015 c |
1.06 |
1.02 |
1 |
0.98 |
EA-900 |
|
0.85 d |
0.89 |
0.86 |
0.84 |
0.83 |
BHT-100 |
|
0.75 d |
0.77 |
0.77 |
0.75 |
0.74 |
BHT / EA |
|
|
1.762d |
1.395c |
1.15b |
0.9783a |
Days average |
Hexane solvent extract peroxide index
The effect of extracts obtained from hexane solvent on crude oil soybean oxidation stability showed that just concentrations above 600 ppm prevent accelerate soybean oil corruption. The results also showed that the concentrations of the extract obtained from the hexane solvent have no effect of intensifying the participants with BHT (Figure 4).
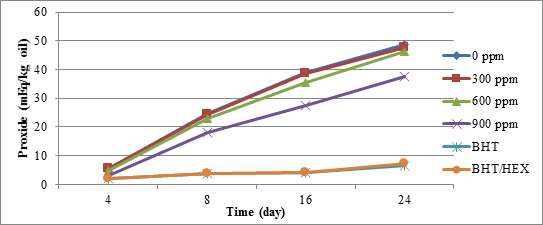
Figure 4. Soybean oil peroxide value changes over time in various amounts of the quinces peel extracts with hexane solvent
Similarly, during the examination of antioxidant ability of cinnamon extract obtained from the acetone and methanol on stability of sunflower oil, the results showed that with increasing the extract concentration, their antioxidant effect on soy bean oil has increased. However, acetone extract showed better performance than that of acetone methanol extract [15]. So that 0.1 percent concentration of that as after synthetic antioxidant TBHQ. This was claimed to be due to the presence of more phenolic compounds in cinnamon extract obtained from acetone.
Thiobarbituric acid index
As seen in Figure 5, extracts obtained by hexane solvent from the quince's peel at concentrations below 600 ppm, have no effect on the oxidative stability of crude soybean oil. And there were no significant changes in the concentration of thiobarbituric index compared to reference sample, within 24 days. However, higher concentrations of 900 ppm up to day 16 could partially prevent the creation of aldehyde compounds in soybean oil but this amount increased up to day 24. No resonance effect produced when using hexane solvent extract of the quince's peel was not observed with the synthetic antioxidant BHT.
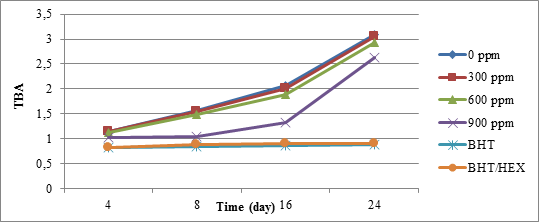
Figure 5. Thiobarbituric acid soybean oil index changes over time in different amounts of extracts derived from quince’s peel with hexane solvent
As shown in the table 3 and 4, changes in the peroxide index and TBA value at zero concentration (control) and 300 ppm did not have a significant difference. Also, the difference between the levels of peroxide and TBA in samples containing BHT and its mixture with quince peel extract was not significant.
Table 3. Compares the average peroxide index and different concentrations of quince’s peel extract with hexane solvent within days (5%)
|
Extracts average |
24 days |
16 days |
8 days |
4 days |
Treatments |
|
29.45 a |
48.6 |
39 |
24.6 |
5.6 |
0 |
|
29.02 a |
47.7 |
38.5 |
24.3 |
5.6 |
HX-300 |
|
27.35 b |
46.1 |
35.6 |
22.8 |
4.9 |
HX-600 |
|
21.63 c |
37.6 |
27.6 |
18.2 |
3.1 |
HX-900 |
|
4.07 d |
6.5 |
4 |
3.9 |
1.9 |
BHT-100 |
|
4.2 d |
7.1 |
4 |
3.8 |
1.9 |
BHT / HX |
|
|
32.26 d |
24.78 c |
16.26 b |
3.83 a |
Days Average |
Table 4. Compares the average index thiobarbituric and different concentrations of quince’s peel extract with hexane solvent within days (5%)
|
Extracts average |
24 days |
16 days |
8 days |
4 days |
Treatments |
|
1.97 a |
3.1 |
2.06 |
1.56 |
1.15 |
0 |
|
1.94 a |
3.05 |
2.01 |
1.56 |
1.15 |
HX-300 |
|
1.86 b |
2.94 |
1.89 |
1.49 |
1.13 |
HX-600 |
|
1.51 c |
2.63 |
1.32 |
1.05 |
1.02 |
HX-900 |
|
0.85 d |
0.89 |
0.86 |
0.84 |
0.83 |
BHT-100 |
|
0.88 d |
0.9 |
0.9 |
0.89 |
0.83 |
BHT / HX |
|
|
2.252 d |
1.506 c |
1.228 b |
1.02 a |
Days Average |
Compare two solvents performance
To assess the value of solvents used in the extraction of effective extract in soybean oil stability, the average values obtained from the peroxide value and thiobarbituric acid in various concentrations were compared. According to Figure 6, it can be observed that extracts obtained via ethyl acetate solvent have been more effective in preventing corruption oxidative crude soybean oil compared to hexane solvent.

Figure 6. Compares ethyl acetate and hexane solvent extract of the bark on the stability of soybean oil based on peroxide value
The reason for this could be due to the higher polarity of ethyl acetate, which has been more effective in separating phenolic compounds. As the same way, the more effectivity of acetone solvent compared to methanol solvent during extraction of antioxidant extract from cinnamon in preventing the oxidation of sunflower oil has been proven, too [15].
Based on the obtained results, this trend also applies in the case of Figure 7.
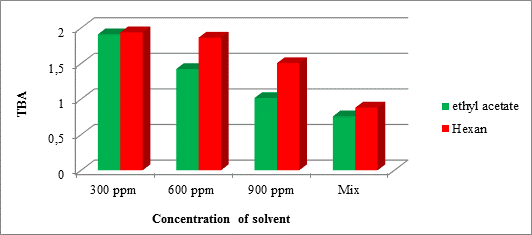
Figure 7 compares ethyl acetate and hexane solvent extract of the quince’s peel in soybean oil stability index based on thiobarbituric acid
Certainly, when polarity of solvent is higher, more phenolic compounds are extracted and its antioxidant capacity increases. Consequently, the formation of secondary oxidation products (as malondialdehyde), which is directly related to the thiobarbituric acid, is reduced.
Conclusion
The results can be summarized briefly as follows: (1) by increasing the extracts concentration, the crude soybean oil stability against oxidation improved. This trend was obvious at concentrations greater than 600 ppm; (2) Over time, the peroxide index values was added, in which values above 600 ppm and synthetic antioxidant BHT can reduce the severity of oxidation over time; (3) Synergistic effect of ethyl acetate extract with synthetic antioxidant BHT was remarkably obvious, while this trend was not obvious in the extracts obtained from hexane solvent; (4) Overall, ethyl acetate extracts was more effective in oxidative stability of crude soybean oil due to the same polarity with phenolic compounds.
References
1. Ranjbar Y., Maghsoudlou M., Ghorbani A.R. Sadeghi Mahounak, The use of pectinase and ethanol treatment for lycopene purity and yield enhancement extracted from tomato peel, EJFPP Persia, 2010, 1 (3), p. 37-50.
2. Rozzi N. L., Kingh R.K., Vierling R. A. and Watking B.A., Supercritical fluid extraction from tomato processing byproduct, J. Agric. Food Chem., 2002, 50, p. 2638- 2643.
3. Abootalebian M., Extraction of phenolic components from mint, bee balm and basil leafs and comparison of their antioxidant power on sunflower oil, M.Sc. Dissertation, faculty of agriculture, Isfahan Industrial University, Iran, 2006.
4. Heli R., Lundy S., Kalicki B., Lutein, Pennington Biomedical Research Centre, Pennington Nutrition Series, 3, 2005 p. 1-4.
5. Alinejad S., Esmaielzadeh Kenari R., Bolandi M., Antioxidant effectiveness of methanol extract from persimmon peels on preservation of sunflower oil during storage, Innovation in Food Science and Technology (J. of Food Science and Tech.), Special Issue, 2013.
6. Tzanakis E., Kalogeropoulos T., Tzimas St., Chatzilazarou A., Katsoyannos E., Phenols and antioxidant activity of apple, quince, pomegranate, bitter orange and almond-leaved pear methanolic extracts, E-Journal of Science & Technology (e-JST), 2006, 1 (3), p. 16-28.
7. Mahmoodi F., Hakimzadeh V., Optimization of antioxidant extraction from quince peels based on antioxidant power and Lutein quantity, M.Sc. Dissertation, Islamic Azad University, Quchan Branch. Iran, 2016.
8. Rojas G., Levaro J. Tortoriello J. and Navarro V., Antimicrobial evaluation of certain plants used in Mexican traditional medicine for the treatment of respiratory disease, J. Ethnopharmacol, 2001, 74, p. 97-101.
9. Sheykhzadeh M., Hakimzadeh V., Abedi Ghanbarabad M., Carotenoids extraction optimization of lutein-based banana Peel, Journal of Applied Environmental and Biological Sciences, 2015, 4 (11S), p. 213-217.
10. Naghavi Azad A., Hakimzadeh V., Golmakani E., Phenolic contents and antioxidants activity from aerial parts of phlomis herba-venti L. subsp. Kopetdaghensis, Journal of Applied. Environmental and Biological Sciences, 2015, 4 (11S), p. 54-58.
11. Fatemi H., Mirahmadi F., Sahari M., Effect of green Tea extract on the inhibition of sunflower oil oxidation, 2006, IJFST Persia, 2 (4), p 61-70.
12. Firestone D., Official methods and recommended practices of American oil chemists’ Society, (Method Cd 8-53 and 19-90), 15th ed. Washington, DC, USA: AOAC, 1990, 1.
13. Chun S.S., Vattem D.A., Lin Y.T. and Shetty K., Phenolic antioxidant from donal oregano (origanum vulgare) with antimicrobial activity against helicobacter pylori, Process biochem., 2005, 40 (2), p. 809-816.
14. Mohagheghi S.A., Poorazarang, H., Elhamirad, A.H., Dezashibi, Z., Hematyar, N. Extraction of phenolic compounds from potato peel ( Ramus variety ) with solvent and ultrasound-assisted methods and evaluation of its antioxidant activity in soybean oil, JFST Spring, 2011, 8 (1), p. 81-91.
15. Ghavami M., Kamaliroosta L., Gharachorloo M., Azizinezhad R., Isolation of cinnamon extract and assessing its effect on the stability of sunflower oil, Iranian Journal of Nutrition Sciences & Food Technology Persia, 2011, 6 (1), p. 13-22.