Engineering, Environment
Enhancing the heating properties of agricultural waste briquettes
Olatunde Ajani OYELARAN*1, Bayode Julius OLORUNFEMI1, Olawale Monsur SANUSI1, Adeyinka Oke FAGBEMIGUN 2, Olusegun Abiodun BALOGUN3
1 Department of Mechanical Engineering, Federal University, Oye-Ekiti, Nigeria
2 Department of Mechanical Engineering, Federal University Technology, Akure, Nigeria
3 Department of Mechanical Engineering, Federal University Agriculture, Abeokuta, Nigeria
E-mail(s): ajanioyelaran@gmail.com1; bayode.olorunfemi@fuoye.edu.ng2; sanuthwale@gmail.com3 ; yinkafabs@gmail.com4; segunmitee2011@yahoo.com
* Corresponding author, phone: +2348028253912
Received: February 07, 2018 / Accepted: May 23, 2018 / Published: June 30, 2018
Abstract
The amount of energy available for consumption is a criterion for economic development of any nation. The necessity to develop alternative energy sources for fossil fuel is evident due to its scarcity, increase in price and non-renewability. The development of energy from biomass is one area among the various energy alternatives that has considerable promise and is receiving attention. The study investigated the effect ultimate and combustion characteristic of homogenous and composite briquettes produced from three agricultural residues in the ratio of 50:50. The results obtained shows that carbon and hydrogen content of composite briquettes are higher than their corresponding homogenous briquettes. The increased in the carbon and hydrogen content led to increase in calorific value of composite briquettes which ranges between 18.77 and 19.28 MJ/kg while for homogenous briquettes is between 17.71 and 19.09 MJ/kg. A graph of calorific value against total hydrogen and carbon contents shows that the calorific values of the briquettes is not linearly related to their total hydrogen contents but directly related to their total carbon content. The coefficient R2, which represent the correlation between calorific values and total carbon and hydrogen contents were calculated as 0.9858 and 0.0365 respectively. The combustion characteristics also shows considerable advantage of composting agricultural waste with thermal efficiency and combustion rate of composite briquettes ranging between 25.25 and 31.69% and 1.63 and 1.71 g/min respectively while that of homogenous briquettes ranges between 19.67 and 23.67% and 0.189 to 0.225 g/min respectively. It could be concluded that production of composite briquette of agricultural waste should be encouraged.
Keywords
Biomass, Composite; Homogenous; Ultimate analysis; Combustion; Solid Fuel
Introduction
Billions of tons of agricultural residue are generated globally every year. This massive quantity of biodegradable wastes can be converted to a large sum of energy and raw materials. As raw materials, biomass wastes have attractive potentials for large-scale industries, cottage enterprises and household utilization Biomass is an attractive energetic source, since it is cheap, renewable and available in most parts of the world. Biomass in all its forms currently provides about 1250 million tonnes of oil equivalent of primary energy which is about 14% of the world’s annual energy consumption [1]. Demirbas [2], wrote that an average majority of biomass energy is produced from wood and wood wastes (64%), followed by solid waste (24%), agricultural waste (5%) and landfill gases (5%). Biomass is the only renewable resource with the potential to produce power, fuels, and chemicals. It is our hope that, with time biomass can replace fossil fuels. Among renewable resources for energy generation, biomass is the least cost alternative. The utilization of lignocellulosic biomass for energy usage has considerably increased. Due to the varied natures of biomass materials, their properties usually range and show different behaviours in thermal processes. The key properties that offer information about a fuel are heating values, proximate (determination of moisture, ash, volatile and fixed carbon content), ultimate analysis (C, H, N, S and O composition) and ash composition, The information on biomass properties enables the forecast of environmental effects and technical parts related to thermal processes. Hence, thermal decomposition behaviour is an outcome of the biomass physicochemical characteristic, which gives valuable information concerning features of the complex reactions that occur throughout the pyrolysis of the biomass. Chirchir et al., [3] wrote that the determination of the calorific values and percentage of C, H, N, S and O of biofuels are important in considering their suitability as environmentally safe energy sources and their potential to produce a particular bio-energy that can be used for industrial and domestic process heat. They also concluded that the estimation of high heating value from the elemental composition of fuel is also important in performance modelling calculations on thermal system.
Composite agricultural waste briquettes may exhibit variation in composition and fuel characteristics, when compared with homogenous agricultural waste briquettes. Therefore the knowledge of their ultimate analysis and heating values has become vital. Briquetting is presently a common practice in developing countries. Briquetting is one of several compaction technologies to form a product of higher bulk density, lower moisture content, and uniform size shape, and material properties [4]. Tabare et al., [2000] also noted that the use of agricultural and forest wastes as well as industrial by-products for production of briquettes are increasing. Some authors worked on the production of briquettes without binder from groundnut shell and waste paper admixture [6], cotton stalks [7] and straws of colza, [8]. Tripathi et al., [9] wrote that briquetting of raw agricultural residues without binder is now a common practice in India. The value of the proximate and ultimate analysis is used to evaluate the fuel value of the raw biomass material, which provides an approximation of the ash handling requirement which describes the burning characteristics.
International Energy Agency, in her report estimated total energy consumption in Nigeria in 2009 was approximated to about 4.6 EJ or 111MTOE [10]. Traditional biomass (wood fuel and charcoal) accounted for 85% of total energy consumption which has contributed to desertification, deforestation and erosion in the country. This high percentage share of biomass represents its use to meet off-grid heating and cooking, mainly in rural areas and by the urban poor. It has been estimated that about 80% of Nigerian households living in the rural and urban areas use wood fuel and charcoal for cooking and heating [11]. The use of wood fuels and other environmental degrading forms of energy in developing countries like Nigeria is aggravated by unaffordability and inaccessibility of conventional forms of energy like electricity by both urban and rural dwellers. This over dependence on wood for both cooking is putting the nation’s dwindling forest under undue stress which is resulting into serious deforestation and desertification. The effect of this could translate subsequently to serious impact on the ecosystem like climate change, agriculture and water resources, if no serious action is taken.
The physicochemical characteristics of biomass are responsible for its attractiveness as a source to harnessed energy [12]. Disposal of biomass wastes, produced in different agro-industrial activities, is normally an environmental problem. A key for such condition is the utilization of these residues for the production of energetic solid bio-fuel by increasing their proximate and ultimate properties of biomass. A lot of studies in different countries have being conducted for the assessment of availability of residual biomass. Scarlat et al. [13] assessed the availability of residual biomass of agricultural and forest crops suitable for bioenergy production in Romania. Crop yield, variation multi-annual yield, environmental and economic constraints and competitive uses were taken into account to estimate agricultural residues. A similar study was developed by Shonhiwa, [14] who explored the magnitude of biomass available for energy production using thermochemical conversion technologies in Zimbabwe. On the other hand, Iye et al., [15] studied the availability of agricultural residues in Nigeria, Oyelaran et al., [16] examined characterization of briquettes produced from groundnut shell and waste paper admixture. In Nigeria, several studies were undertaken to characterize residues from agriculture, animal, forestry and municipal solid waste in order to assess its energy potential [17]. As a result of its location in the tropics, Nigeria has comparative advantages in the production of agricultural and forest biomass and its availability are sufficient to satisfy the energy demands of the country.
The main objective of this research work is to investigate the effect ultimate and combustion characteristic of homogenous and composite briquettes produced from three agricultural residues in the ratio of 50:50 with the aim of discovering if there are considerable effects on them, when compared with homogenous agricultural waste briquettes.
Material and method
Sampling of biomass materials
Three (3) selected agricultural residues (Corn cob, groundnut shell and banana leaves) locally produced in Nigeria’s farmlands were collected from farmlands and local community around Kano and Ikole-Ekiti in Nigeria. They were kept under room temperature and used in this experimental work without any pre-treatment so as to represent the actual situation (condition) by which they are used as fuel.
Preparation of the briquette samples
The preparation of the briquette samples was carried out using the steps shown in Figure 1.
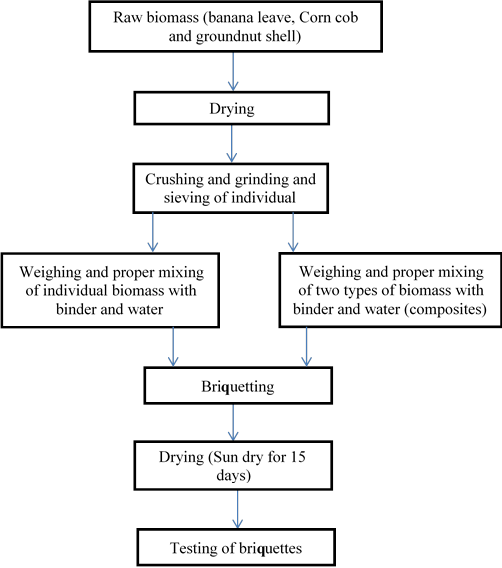
Figure 2. The basic flow process for briquettes production
The briquettes were produced using a manually operated hydraulic briquetting machine. Briquettes of the various compositions were shaped with a precise amount of starch based on the whole mass of the blend was used as the briquette binder as shown in Table 1. Throughout the production, specific quantity of water was added to the blend to attain homogeneity and the pressure was maintained at 5MPa throughout the production time, after the production of these briquettes it was sun dried for 15 days before study.
Table 1. The briquette produced with the ratio of their percentage compositions
|
Sample |
Composition of briquette studied |
|
B |
100% banana leave |
|
C |
100% corn cob |
|
G |
100% groundnut shell |
|
CG |
50% corn cob and 50% groundnut shell |
|
CB |
50% corn cob and 50% banana leave |
|
GB |
50% groundnut shell and 50% banana leave |
Determination of ultimate analyses of the individual and composite agricultural waste briquette
The ultimate analyses of the individual and composite agricultural waste briquettes were determined (which include carbon, hydrogen, oxygen, nitrogen and sulphur content). The determination of their percentage compositions were carried out using standard methods. The percentage oxygen content of the individual composite agricultural waste briquette was determined by difference while the heating value was determined using Gallenkamp Ballistic Bomb Calorimeter.
Determination of ash content (AC)
About 2g of finely ground oven dried sample was placed in a porcelain crucible and weighed, W1, before it was transferred into a preheated muffle furnace set at temperature of 900°C. The furnace was left on for about an hour after which the crucible with the content was transferred to the desiccators and allowed to cool. The crucible with its content was reweighed, Wf and the weight of the empty crucible was Wc, the ash content (% dry basis), was given as Eq. (1):
Ash ![]()
![]()
![]() 100
(1)
100
(1)
Where: Wf - final weight of crucible with sample after heating, Wc - weight of empty crucible and W1 - initial weight of crucible with sample before heating.
Determination of carbon and hydrogen contents
Carbon and hydrogen contents biomass feedstock were determined simultaneously by Leibig – Pregl method. 1g of sample flour was placed in a quartz test tube and burned off through the absorbents magnesium percolate to absorb water and sodium hydroxide to absorb carbon dioxide. The amounts of water and carbon dioxide were determined from the difference between the two weightings, one before the other after the absorption of water and carbon dioxide. The percentage of Carbon (%C) and hydrogen (%H) were evaluated thus Eq. (2-3):
% C = ![]() ×
100%
(2)
×
100%
(2)
% H
= ![]() ×100%
(3)
×100%
(3)
Where: a - quantity of CO2, b - quantity of H2O
Determination of nitrogen contents
Nitrogen content of biomass feedstock was determined by Dumas – Pregl method. A 0.2g of the sample flour was mixed with powder of copper oxide in the ignition tube. Air was displaced from the tube by passing through a stream of CO2 until minute bubble appeared in the Nitrogen flow meter filled with about 50% solution of potassium hydrogen. The weighed sample was burned off at between 700°C and 750°C in a gas burner and later burned in an atmosphere of CO2 with the gas cylinder shut off. After ignition, the combustion product was displaced with carbon dioxide into the nitrogen flow meter. The percentage Nitrogen (%N) content was determined by the Eq. (4):
%N = ![]() ×
100%
(4)
×
100%
(4)
Where: V - volume of Nitrogen in the Nitrogen flow meter, 1.097 = mass of 1 ml of Nitrogen at the test tube.
Determination of sulphur content
A 1g of biomass feedstock sample flour was wrapped in a filter paper free from ash and it was secured in the platinum wire seal into a glass rod held fast to the stopper of a flask filled with Oxygen. The weighed sample was ignited in filter and inserted in the flask immediately, and the flask was plugged with the stopper. The product was absorbed with a mixture of water and Hydrogen peroxide to oxidize the combustion product immediately. The combustion product was titrated with a solution of Barium percolate in the presence of the indicator Toron with a PH value of 4.5. The percentage of sulfur was found by the Eq. (5):
%S = ![]() ×100% (5)
×100% (5)
Where: T - titre of Ba (CO4)2 solution, V - volume of Ba (CO4)2 solution.
Determination of oxygen content
The percentage oxygen content was determined as follows, Eq. (6) [18]:
% Oxygen = 100 – (C+H+N+S) (6)
Where: C - % carbon content in the biomass fuel, H - % hydrogen content in the biomass fuel, N - % nitrogen content in the biomass fuel, S - % sulphur content in the biomass fuel.
Determination of combustion properties of individual and composite agricultural waste briquette
In this study, combustion analysis was conducted to understand the combustion characteristics of the briquette fuel, flame propagation rate, afterglow time and water boiling test was carried out to replicate cooking conditions when briquette fuel is utilized. The combustion performance of each briquette produced was recorded. Tables for the combustion parameters were made to ascertain the combustion performance of each of the briquette.
Determination of calorific value of the briquettes produced
Calorific value of the sample was determined using Gallenkamp Ballistic Bomb Calorimeter according to ASTM E711-87 [19].
Determination of ignition time of the briquettes produced
Ignition time was determined according to Onuegbu et al., [20]. Each briquette was ignited by placing a Bunsenn burner on a platform 4 cm directly beneath. Bunsen burner was used to ensure that the whole of the bottom surface of the briquette was ignited simultaneously after adjusting it to blue flame. Caution was taken to avoid flame spread in the transverse directions. The burner was left in until the briquette was well ignited and had entered into its steady state burn phase.
Determination of combustion rate of the briquettes produced
Burning time was obtained by observing the mass changes recorded on mechanical balance and also by using stop watch. It is the time for the biomass combustion to be completed. With known amount of total burnt briquette and burning time, average combustion rate can be calculated using the following Eq. (7) [21].
CR = ![]() (7)
(7)
Determination of thermal fuel efficiency of the briquettes produced
Thermal fuel efficiency (![]() of the energy was calculated according to
Oladeji, Eq. (8) [22]:
of the energy was calculated according to
Oladeji, Eq. (8) [22]:
![]() (8)
(8)
The numerator gives the net heat supplied to the water, while the denominator gives the net heat liberated by the fuel.
Where: PHU - percentage heat utilized (%); P - power output (KW); SFC - specific fuel consumption (kg of fuel/kg of water); B.R - burning rate (kg/s); mw - mass of water in the pot (kg); T0 - initial temperature of water (K); T1 - boiling temperature of the water (K); mc - mass of water evaporated (kg); L - latent heat of evaporation (KJ/kgmol); mf - mass of fuel burnt (kg); Ef - calorific value of the fuel (KJ/kg); t - time taken to burn fuel (s).
Results and discussion
The results of the ultimate analysis of the individual and composite
The results of the ultimate analysis of the individual and composite briquettes are shown in Table 2.
Table 2. Ultimate analysis and the heating values of briquettes
|
Sample |
C% |
H% |
N% |
S% |
O% |
Ash% |
CV (MJ/kg) |
|
C |
46.32 |
3.44 |
0.36 |
0.88 |
49.00 |
5.12 |
17.71 |
|
G |
47.43 |
4.52 |
1.31 |
0.13 |
46.61 |
5.47 |
18.53 |
|
B |
44.28 |
6.23 |
0.80 |
0.21 |
48.48 |
10.16 |
19.09 |
|
CG |
48.23 |
7.13 |
0.51 |
0.30 |
43.23 |
5.51 |
19.28 |
|
CB |
47.11 |
6.98 |
0.38 |
0.83 |
44.70 |
8.66 |
18.77 |
|
GB |
47.62 |
7.11 |
0.81 |
0.21 |
44.25 |
9.17 |
19.14 |
It can be seen from the table 2 that, the carbon and hydrogen content of composite corn cob and groundnut shell briquettes are higher than that of composite corn cob and banana leave briquettes and composite banana leave and groundnut shell briquettes. However, comparing the carbon and hydrogen content of homogenous agricultural waste briquette with those of the composite briquettes those of the composite are higher than their corresponding individual briquette. As shown in Table 2, the trend in the percentage nitrogen content of the briquettes have no define pattern but can be concluded that the composite briquettes achieved some reduction in the nitrogen content except for groundnut shell and banana leave composite briquette that has small increase in the nitrogen content. The low content of nitrogen in the briquettes whether homogeneous or composite result in low emission of NOx. The nitrogen which comes with air for combustion of agricultural waste does not oxide since they oxides at a temperature of about 1500 0C (Nag, 2001) and the combustion temperature of agricultural waste briquette are usually less than 1500 0C. The percentage sulphur content in the homogenous and composite agricultural waste briquettes are low as shown in Table 2. There are no significant increases when the sulphur content of the homogenous and composite briquettes is compared. This means that, the low sulphur content in the briquettes will result in low emission of its oxides when the briquettes are burnt. The oxygen content of the composite briquettes is less than that of homogenous briquettes as seen in Table 2. Han, [23] wrote that higher amount of oxygen may lead to increase in NOX and SOX emission. Accordingly, less emissions of the oxide of nitrogen and sulphur from composite briquette is expected, compare to those from homogenous briquettes. The ash content of composite corn cob and banana leave and that groundnut shell and banana leave briquettes were found to be higher than that of homogenous corn cob and groundnut shell briquettes. While that of composite corn cob and groundnut shell briquette is in an average range of the ash content of homogenous groundnut shell briquette and a little higher than that of homogenous corn cob briquette as shown in Table 2. Demibras, [24] noted that high ash content reduces ignitibility of the fuel briquettes.
Relationship between calorific value (CV) and total hydrogen/carbon contents (%wt) of the samples
The calorific value is measured directly with bomb calorimeter. A graph of calorific value against total hydrogen and carbon contents is plotted. From the data shown in Tables 2 the trend of data plotted in Figure 1 and 2, it is apparent that the calorific values of the briquettes is not linearly related to their total hydrogen contents but directly related to their total carbon content, i.e. the higher the carbon content of biomass, the higher the heat energy to do a useful work.
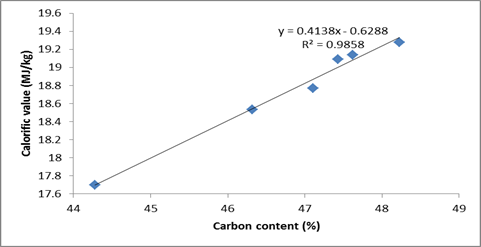
Figure 1. Calorific value against carbon contents
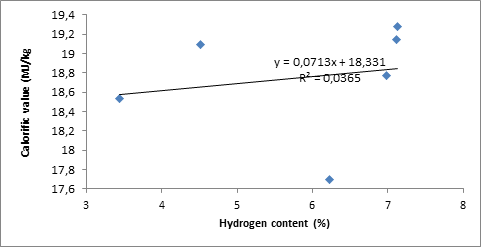
Figure 2. Calorific value against total hydrogen contents
The coefficient R2, which represent the correlation between calorific values and total carbon and hydrogen contents were calculated as 0.9858 and 0.0365 respectively. These calculated values show that the relationship between calorific values and total carbon contents is positive/and directly while for hydrogen content there is no correlation. These results are in good agreement with the theoretical equations derived by Parikh [24] that the high heating value of biomass materials is a very strong function of its fixed carbon content. The fixed carbon content represents the amount of combustible material present in the char residue after loss of moisture and volatile matter. This proved that the gross calorific value is most influenced by the composition of briquette The calorific value of the briquettes studied compares well with that reported by RETSASIA, [25] 19,534 kJ/kg for briquettes from a mixture of palm kernel cake (PKC) and sawdust and 18,936 kJ/kg for sawdust of some hardwood species [26]. This value is also compares with the heating values of briquettes produced from corncob, groundnut shell, melon shell, cassava and yam peels and were found to be 20,890, 18,634.34, 21,887, 12,765, and 17,348 kJ/kg, respectively Oladeji [27]. These energy values obtained for the whole samples are sufficient enough to produce heat required for household cooking and small scale industrial cottage applications. The calorific value of the briquettes is within the acceptable range for commercial briquette fulfilling the minimum requirement of calorific value for making commercial briquette (>17500 J/g), as stated by DIN 51731[26, 28].
The carbon and oxygen content is important in biomass energy analysis. High Oxygen content tends to lower the calorific value while high carbon content tends to form high grade biomass fuel. It is evident from Table 2 and 3 that high carbon contents contributes to the calorific value positively and high oxygen content is contributes negatively. It is therefore important in biomass conversion routes to deoxidize the biomass in order to increase the heating value. This was achieved by composite application. The measured percentage H, N and S contents are generally much lower to that of hydrocarbon fuels and oxygen content is high to that of hydrocarbon. The total nitrogen varies in the range 0.36 -1.31(wt %) and the total sulphur content ranges between 0.13 - 0.91 (wt %). Both nitrogen and sulfur content are not important in biomass combustion. They tend to increase the release of toxic gases that are either irritants (NOx, SO2, aldehydes and acrolein) or asphyxiants (HCN) which may cause adverse effect to living organisms.
The results of the Combustion Characteristic of the individual and composite
A low ignition time briquette indicates safety concerns during storage, the risk of ignition and fire is increased in briquettes with low ignition time. Thus, caution must be taken to keep briquettes free from sparks and extreme heat. The ignition time is a function of the volatile matter and particle size. The higher the volatile matter the higher the ignition time since more time is taken to burn off the volatiles before combustion. Also the larger the particle sizes the higher the ignition time. The ignition time ranges between 64.43 and 88.54 minutes. The recorded lowest ignition time recorded for corn cob and banana leave composite briquettes could be attributed to high porosity exhibited between inter- and intra-particles which enable easy percolation of oxygen and outflow of combustion briquettes due to low bonding force.
The obtained result for thermal fuel efficiency of briquettes ranges between 19.67 – 31.69% as shown in Table 3.
Table 3. Results of combustion properties of briquettes
|
Sample |
|
Ignition Time (min) |
Combustion rate (g/min) |
|
B |
19.67 |
88.54 |
0.225 |
|
C |
21.79 |
67.23 |
0.201 |
|
G |
23.67 |
72.54 |
0.189 |
|
CG |
25.25 |
68.37 |
0.171 |
|
CB |
31.25 |
64.43 |
0.169 |
|
GB |
31.69 |
87.14 |
0.163 |
The results reveal that composite briquettes have higher thermal efficiency than their corresponding homogeneous briquettes. This shows that the groundnut shell-banana leave briquette would be the best fuel for domestic cooking. This briquette would require the lowest quantity in kilograms to bring an amount of water to boil and evaporate.
Combustion rate is one of the important characteristics to confirm the quality of briquettes, it is the amount of a material that undergoes combustion at a period of time [21]. The result of the combustion rate of briquettes is shown in Table 3. The lowest combustion rate of 0.163 g/min was recorded in groundnut shell and banana leave composite briquette, with homogenous banana leave briquette recording the highest of 0.225 g/min. Combustion rate has a significant effect on briquette application. A briquette with high combustion rate implies that more briquettes will be required in combustion as they burn off readily. The combustion rate in this study which ranges between 0.163 and 0.225 g/min is comparable with that reported by Oyelaran et al, [21] for tannery solid waste briquette which ranges between 0.171 and 0.217 g/min and lower than that reported by Islam et al. (2014) of briquette from Coir Dust and Rice Husk Blend which varies between 0.789-0.945 kg/hour and 0.43 g/min reported by Nasrin et al, [29] for palm briquette.
Conclusions
Composite solid fuel was produced from three homogenous agricultural waste in a ratio of 50:50 and processed through briquetting method using cassava starch as binder. The produced briquettes were subjected ultimate analysis and combustion test with a view of assessing their combustion quality and suitability. The results obtained shows that composite briquettes have enhanced Carbon and Hydrogen contents as well as heating value, compared to their individual homogenous briquettes as evident in this research work. The produced briquettes were found to meet the requirement for household cooking, heating and for used in cottage industries.
References
1. Weither J., Saenger M., Hartge E.U., Ogada T., and Siagi Z., Combustion of agricultural residues, Progress in Energy and Combustion Science, 2000, 26, p. 1-27.
2. Demirbas A., Mechanisms of lignification and pyrolysis reactions of biomass, Energy Convers. Mgmt., 2000, 41, p. 633-646.
3. Chirchir D.K., Githeko J.M. and Nyaanga D.M., Effect of binder types and amount on physical and combustion characteristics, International Journal of Engineering Science and Technology, 2013, 2, p. 12–20.
4. Making Charcoal: The Retort Method. Volunteers in Technical Assistance, 1815 North Lynn St, Suite 200, Box 12438, Arlington, VA 22209. 1980. ISBN 0-86619-07.
5. Tabares J.L.M., Ortizb L., Granadaa E. and Viar F.P., Feasibility study of energy use for densificated lignocellulosic material (briquettes). Fuel, 2000, 79, p. 1229-1237.
6. Oyelaran O.A., Development of a motorized Biomass briquetting Machine, Ph.D thesis Department of Mechanical Engineering, Federal University of Agriculture, Abeokuta, Nigeria. 2014.
7. Abasaeed A. E., Briquetting of carbonized cotton stalk, Ener., 17 (9), p. 877-82.
8. Bartel Y., Energizing valorisations of some agriculture products by densification, Notebooks Agris., 1992, 6 (3), p. 208-12.
9. Tripathi A.K., Iyer P.V.R. and Kandpal T.C., A techno-economic evaluation of biomass briquetting in India, Biom. and Bioe., 1998, 14 (5-6), p. 479-88.
10. IEA, Energy balance for Nigeria. OECD/IEA. http://data.iea.org. Accessed 1st September 2012.
11. Sambo A.S., Renewable energy electricity in Nigeria: The way forward, Renewable Electricity Policy Conference held at Shehu Musa Yarádua Centre, Abuja, 2006, p. 11-12.
12. Tuates Jr.A.M., Bio-energy in the Philippines: status and utilization. Paper presented during the Bioenergy Information Day. Frankfurt, Germany, 2016.
13. Scarlat N., Blujdea V., Dallemand J., Assessment of the availability of agricultural and forest residues for bioenergy production in Romania, Biomass and Bioenergy, 2011, 35, p. 1995-2005.
14. Shonhiwa C., An assessment of biomass residue sustainably available for thermochemical conversion to energy in Zimbabwe, Biomass and Bioenergy, 2013, 52, p. 131-138.
15. Iye E., Bilsborrow P., Assessment of the availability of agricultural residues on a zonal basis for medium- to large-scale bioenergy production in Nigeria, Biomass and Bioenergy, 2013, 48, p. 66-74.
16. Oyelaran O.A., Bolaji B.O., Waheed M.A. and Adekunle M.F., Characterization of briquettes produced from groundnut shell and waste paper admixture, Iranica Journal of Energy and Environment, 2015, 6 (1), p. 34-38.
17. Jekayinfa S.O. and Omisakin O.S., The energy potentials of some agricultural wastes as local fuel materials in Nigeria, Agricultural Engineering International: the CIGR Ejournal, 2005, 7, Manuscript EE 05 003.
18. Anonymous, Annual Book of ASTM Methods, 1992, 5, D4239.
19. ASTM E 711- 87.
20. Onuegbu T.U., Ekpunobi U.E., Ogbu I.M., Ekeoma M.O. and Obumselu F.O. Comparative studies of ignition time and water boiling test of coal and biomass briquettes blend, International Journal of Research & Reviews in Applied Sciences, 2012, 7, p. 153–159.
21. Oyelaran O.A., Sani, F.M., Sanusi, O.M., Balogun, O. and Fagbemigun, A.O., Energy Potentials of Briquette Produced from Tannery Solid Waste Makara, J. Technol. 2017, 21 (3), p. 122-128 doi: 10.7454/mst.v21i3.3091.
22. Oladeji J.T., The effects of some processing parameters on physical and combustion characteristics of corncob briquettes, Ph.D. Thesis, Department of Mechanical Engineering, Ladoke Akintola University of Technology,Ogbomoso, Nigeria, 2011.
23. Han L.V., Co-firing of rice husk for electricity generation in Malaysia, B. Eng; Dissertation, Faculty of Engineering and Surveying, University of Southern Queensland, 2004.
24. Parikh J., Channiwala S.A. and Ghosal G.K., A correlation for calculating elemental composition from proximate analysis of biomass materials, Fuel, 20017, 86 (12-13), p. 1710-1719.
25. RETSASIA, Results earlier reported for sawdust briquette and torrefied wood [cited 2012 Oct 1]. (Available: www.retsasia.ait.ac.th/publication /WRERC2005/RONAST-WRERC05 (2005).
26. Oyelaran O.A., Bolaji B.O., Waheed M.A. and Adekunle M.F., An experimental study of the combustion characteristics of groundnut shell and waste paper admixture briquettes, KKU Engineering Journal, 2015, 42 (4), p. 283-286.
27. Oladeji J.T., Comparative study of briquetting of few selected agro-residues commonly found in Nigeria, The Pacific Journal of Science and Technology, 2012, 13 (2), p. 80-86.
28. Yuhazri1 M.Y., Sihombing H., Nirmal U., Lau S., Prak T.P., Solid fuel from empty fruit bunch fiber and waste papers, Part 1: Heat released from combustion test. Global Engineers & Technologists Review, 2012, 2 (1), p. 7-13.
29. Nasrin A.B.Ma, Choo A.N., Mohamad Y.M., Rohaya S., Azali M.H.A., Zainal Z., Oil palm biomass as potential substitution raw materials for commercial biomass briquettes production, American Journal of Applied Sciences, 2008, 5, p. 179-183.