Modelling the products’ yield of used tyre pyrolyzed in fixed bed reactor
Tunde Isaac OGEDENGBE 1*, Olufemi OROYE 2, Adebisi Olayinka AKINOLA 3
1,2,3Department of Mechanical Engineering, Faculty of Engineering, Federal University of Technology, Akure, PMB 704, Ondo State, Nigeria
E-mail(s): 1tioged@yahoo.com/tiogedengbe@futa.edu.ng; 2olufemioroye@gmail.com; 3aoakinola@futa.edu.ng
* Corresponding author phone: +2348038073724:
Received: 06 February, 2018 / Accepted: May 24, 2018 / Published: June 30, 2018
Abstract
Pyrolysis of used tyre to obtain by-products namely char, pyro-oil and pyro-gas was carried out to investigate the main and interaction effect of pyrolysis temperature and feedstock sizes on the products yield. A full factorial experimental approach was adopted to conduct the pyrolysis experiments. The values of the process parameters used for the experiments were varies at three levels and combined to give nine experiments. Each of the nine experiments were repeated thrice to arrive at a total of twenty seven experimental set up which were carried out and assessed using Analysis of Variance (ANOVA). Subsequently, models were formulated and validated for estimating the pyrolysis products yield in respect of the operating parameter considered. Result shows that the main effect of change in pyrolysis temperature and feedstock size on all products yield was statistically significant while the effect resulting from the interaction between the process parameters is not. The models formulated for predicting the products yield were able to adequately estimate the percentage yield obtained from experiments, using the values of the pyrolysis temperature and feedstock size, with a correlation coefficient of 90.00 %, 78.63 % and 95.11 % for char, pyro-oil and pyro-gas yields respectively.
Keywords
Analysis of variance; Modelling; Pyrolysis; Tyre; Waste to wealth
Introduction
Waste is an unavoidable by-product of human activities. The two fundamental classes known are general wastes and hazardous wastes. General wastes include domestic, industrial and institutional waste while hazardous wastes include explosives, flammable liquids and solids as well as corrosives waste. Used tyres belongs to the general wastes category which gives rise to land filling as well as environmental and health challenge [1]. Used tyres present formidable disposals problem. The same property that makes them desirable as tyres also makes their disposal and reprocessing difficult. Tyres are almost immune to biological degradation; this is so because they are designed to withstand hash conditions such as exposure to ozone, friction, light and bacteria [2]. Hence, the indiscriminate discarding and stockpile of worn out tyres (hereafter referred to as used tyres) is a challenge to safety and environment. It poses three major health and environmental degradation threats namely (a) Pests and Mosquitoes (b) Fire and (c) Space. Disease carrying pest such as rodent lives and breeds in tyre piles likewise mosquitoes due to stagnant rain water collected inside tyres because of its shape and impermeability. This makes eradication and control of pests and mosquitoes program difficult to achieve. Though difficult to ignite, if tyre piles catch fire, it is nearly almost impossible to extinguish which can lead to destruction of the nearby ecosystem and pollution of the environment with its rank emission smell, noxious, and toxic fumes. Also, tyre is of low bulk density and about 75% of its volume is empty space hence occupies larger amount of valuable space in land fill and when its pile up. Therefore, considering the associated problems of disposing used tyre, the most feasible option left is to recycle them and one of the methods which is very useful to achieve this is pyrolysis.
Pyrolysis is a chemical reaction that involves molecular breakdown of larger molecules into smaller molecules in the presence of heat and absence of oxidative agent [3]. It offers ecologically attractive way to decompose used tyres into useful products and energy [4]. Pyrolysis of used tyres would result in the production of three categories of outputs/products namely: (a) solid residue also refers to as char; (b) condensable liquid fraction called pyro-oil and (c) pyro-gas [5].
The solid residue (char) contains carbon black and the mineral matter initially present in the tyre. The pyro-oil is the liquid product of the tyre pyrolysis process general referred to as tyre pyrolysis oil (TPO). TPO has a wide range of potential applications, such as being an alternative to fossil fuel, exhibiting very good performance in combustion, storage, emission and distillation systems, based on its fuel properties reported by existing studies [6-7]. The oil is low in sulphur which is of the range (0.3-0.1%); its gross calorific value is 42.9MJ/kg and it contains aromatic hydrocarbon, alkanes, alkenes, ketones and aldehydes.
Pyro-gas is the gaseous product of the pyrolysis process. It is used as source of fuel for pyrolysis reactor and turbines and as cooking gas for domestic purpose [8]. The calorific value of pyro-gas is of the range 30-40 MJN/m3 which is sufficient to provide energy required for small process plants.
The yield of pyrolysis by products depends on the operating conditions such as temperature, pressure, heating rate, feedstock sizes, reactor type, and heat exchange type [9]. The two factors that govern used tyre pyrolysis products’ yields are : - (a) Environment, such as operating pressure, temperature, resident time and reactor type; and (b) Materials condition which includes age of material, composition, feedstock sizes and type of material [10].
Pyrolysis of used tyres in both laboratory and industrial scale have been investigated with a range of different reactors such as fixed bed batch, closed batch, moving screw bed, rotary kiln, vacuum, conical spouted bed and fluidized bed [11]. The key influence on the used tyre pyrolysis products’ yield is reported to be the type of reactor used which in turn determines the temperature and heating rate; hence it was concluded that pyrolysis reactor is the heart of pyrolysis process. However, it has been reported that fixed bed pyrolysis is more attractive than other thermo-chemical conversion process because of its simplicity and higher conversion of pyrolysis feedstock to its by-products [12]. It was further observed that losses in fixed bed pyrolysis are relatively less than fluidized bed pyrolysis which is more complex hence, fixed bed reactor should be preferred for used tyre pyrolysis.
According to Kyari et al. [13] used tyre pyrolysis process yields were not significantly influenced by the tyre’s brand and its origin. However the study concluded that there are difference in composition of the derived gases and oil from pyrolysis of different brand and types of tyre from different countries which might have resulted from the variation in the composition of the used tyre feedstocks.
The heating rate in a whole tyre feedstock is low due to its lower thermal conductivity as a result of heat not uniformly spreading all through the feedstock size at a time hence, during pyrolysis, heat can only flow to a certain depth at a specified temperature and residence time [6]. Thus the rubber core of larger pieces can’t be completely decomposed resulting in char yield increase and decrease in both liquid and gas yield. Also, Laresgoiti [14] reported that the smaller the feedstock sizes the higher the heating rate and its rapid decomposition due to provision of more reaction surface. The oil vapour produced have enough time for secondary reactions in the reactor this consequently increases gas yield and reduces both the liquid and char yield respectively.
Debalaxmi and Singh [15] conducted pyrolysis on bicycle waste tyre in a semi batch reactor at a temperature range of 450-650oC reported that the pyro-oil yield increased from 19.6% to 49.6% with increase in temperature likewise does the gas yield increase in such a manner while at same time the char yield decreases as the temperature increases. Furthermore, Nhlanhla and Edison [6] reported that at high temperature gas yield dominates the product yield with char loss during the thermal cracking. This is associated with secondary cracking reaction occurring at high temperature which leads to breaking of the liquid molecules into vapour hence, an increase in gaseous yield was observed. Also, Hariram and Mohammed [16] and Balat et al. [17] share the aforementioned view with regard to the relationship between pyrolysis process operating temperature and the process products’ yield.
Hence, rapid decomposition of feedstock occurs at smaller sizes due to high heating rate while at bigger sizes, the heating rate is low due to its lower thermal conductivity [14], also, of all the three pyrolysis products, pyro-gas yields dominate at higher temperature while medium temperature favours production of pyro-oil and char [6]. While process temperature and feedstock sizes have been investigated separately and found to affect the yield of pyrolyzed used tyre products as reported herein from existing studies consulted, there is need to study the two process parameters together and to investigate whether the interaction between the two parameters actually affect the pyrolysis products’ yield with a focus on developing models for predicting products’ yield from the process parameters. This is the subject matter of this study wherein used tyres having different feedstock sizes were pyrolyzed, in a fixed bed reactor, at different temperatures.
The purpose of the study, therefore, is to determine the main effect of each of the operating parameters as well as their interaction effect on the pyrolysis products yield so that the main parameter(s) and/or their interaction that exhibit significant effect on products yield were utilized to develop regression models for predicting the products yield.
Materials and Method
Michelin used tyres were sourced from tyre refurbishing stations in Akure. These were used as feedstock for the pyrolysis process. Figure 1 shows the flow diagram of the working algorithm.
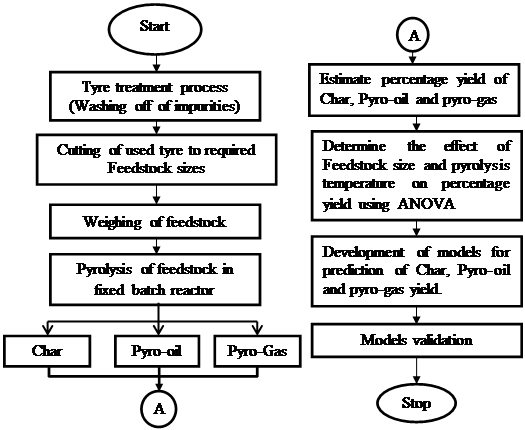
(a) Experimental phase (b) Analysis phase
Figure 1. Flow diagram of the working algorithm
The used tyres were thoroughly washed to remove impurities that can affect the pyrolysis process (see Figure 1 (a)). Then they were shredded into three sets of samples each having a feedstock size with a dimension of 20 x 20 x 6.8 mm, 40 x 40 x 6.8 mm and 60 x 60 x 6.8 mm.
The different sizes into which the feedstock was shredded were weighed and their percentage weight, in respect of a total sample weight of 1 kg to be pyrolyzed, was determined in Eq. (1) as 0.47, 1.84 and 3.28 % respectively. All weight measurements were done using a digital weighing balance.
![]() =
(
=
(![]() )
)![]() (1)
(1)
Where: fs - the percentage weight by feedstock size, Wfp - the weight of one piece of a given feedstock sample and Wfsample - the total weight of the feedstock sample in Eq. (1).
Proximate and ultimate analysis of the used tyre sample was done at the Federal College of Agriculture Research Laboratory, Morr Plantation, Ibadan, and Oyo State Nigeria.
One kilogram of each of the samples having similar sizes of shredded feedstock was pyrolysed at different operating temperature to obtain char, pyro-oil and pyrogas. The various levels of operating temperature considered are 4000C, 5000C and 6000C. Pyrolysis of the used tyres was carried out using a thermos-chemical fixed bed pyrolysis reactor of 17.4 liters capacity which can withstand a pressure of 2.3 MN/m3 and temperature of 12000C [18]. The three samples with different sizes were combined with the three operating temperatures considered using full factorial experimental design approach to obtain nine set of pyrolysis experiments to be conducted with used tyre samples having different feedstock sizes and at different pyrolysis temperature. Each of the nine experiments was repeated three times to give a total of twenty seven pyrolysis experiments conducted randomly.
At the end of each experiment, the product of pyrolysis namely char, pyro-oil and pyro-gas were collected (Figure 1(a)). Thereafter, the study proceeded to the analysis phase (Figure 1(b)) wherein at the first stage the products yield were weighted and recorded for each experiment. The char, pyro-oil and pyro-gas were collected in pan, condensate receiver and gas receiver respectively.
Mathematical analysis
The weight of char, pyro-oil and pyro-gas obtained as products of the pyrolysis process was determined as follows.
Weight of Char
To determine the weight of the char, the weight of the weighing pan denoted as Wp was determine and subtracted from the combined weight of the char and that of the pan denoted as Wpchar using Eq. (2).
![]() (2)
(2)
Weight of Pyro-oil
The condensate receiver (conical flask) containing the condense pyro-oil was weighed when empty and denoted as Wc. Subsequently it was weighed when fill with pyro-oil and denoted as Wcoil. Hence, the weight of the pyro-oil Woil was then obtained from Eq. (3).
![]() (3)
(3)
Weight of Pyro-gas
The pyrogas weight Wgas was obtained from the difference between the gas receiver weights Wr and the weight of the receiver plus the collected gas Wrgas. Hence weight of pyro-gas is obtained according to Eq. (4).
![]() (4)
(4)
Percentage yield
Percentage yield is the ratio of the weight of the yield to that of the feedstock sample in percentage. Having determined the weight of char yield, pyro-oil yield and pyro-gas yield respectively in Eq. (2-4), the percentage char yield (Ƴchar), the percentage pyro-oil yield (Ƴoil) and the percentage pyro-gas yield (Ƴgas) was respectively determined according to Eq. (5-7):
![]() (5)
(5)
![]() (6)
(6)
![]() (7)
(7)
Analysis of variance
The effect of feedstock size and pyrolysis temperature on percentage yield of the char, pyro-oil and pyro-gas was analyzed using analysis of variance (ANOVA). Three conditions were investigated on the basis of which the following hypotheses were considered:
i.) Change in pyrolysis temperature have effect on the pyrolysis product yields or not.
ii.) Change in feedstock sizes have effect on the pyrolysis products yield or not.
iii.) The interaction arising from change in pyrolysis temperature and feedstock sizes have effect on the pyrolysis product yields or not.
The three aforementioned conditions were each considered as null hypothesis Ho when change does not have effect on the pyrolysis product yield. Hence the alternate hypothesis becomes the case when change does have effect on the pyrolysis product yield.
The null hypothesis is rejected and the alternate hypothesis is accepted if the F value calculated (F calculated) on the ANOVA Table is greater the F value obtained from the Table of F-distribution (F Table) and vice versa.
Formulation of model
The process parameters found to have significant influence on any of the pyrolysis products yield were utilized to formulate model for predicting the product yield. The general form of the model considered is a first order multiple linear regression model. The model is as expressed in Eq. (8).
![]() (8)
(8)
Where: Y - the percentage yield of pyrolysis product i, (β0, β1, β2, and β12) are the regression coefficient, X1 and X2 represents the pyrolysis temperature and the feedstock size respectively which used to obtain the pyrolysis product i that is char, pyro-oil or pyrogas.
If the effect of the interaction between the process parameters is not statistically significant on the yield of all the pyrolysis products considered as is the case in this study after applying analysis of variance, it is neglected for estimating the products’ yield. Hence, the third term of Eq. (8) reduces to zero so that only the main effect of the process parameters is considered for the model formulation.
If the pyrolysis temperature represented as T is set to be X1 and the feedstock size represented as S is set to be X2, Eq. (8) becomes
![]() (9)
(9)
The multiple linear regression equation is evaluated using experimented data to obtain the regression/constant coefficients towards formulating the predictive models. This facilitates determination of the best set of the regression coefficients such that the model predicts the products yield for a given set of process parameters as accurate as possible. The data points obtained from initial experiments when applied to Eq. (9) resulted in the regression coefficients given in Table 6 as being obtained for the relevant product yield equation.
Consequently, the model equation for predicting char yield, pyro-oil yield and pyrogas yield is written as given in Eq. (10), (11) and (12) respectively.
Ychar = 59.73 - 0.03039 T - 0.000452 S (10)
Yp-oil = 56.56 - 0.03417 T+ 0.000917 S (11)
Yp-gas = -16.26 + 0.06450 T - 0.000473 S (12)
Validation of model equations
A set of twenty separate experiments were conducted within the range of the values of the operating parameters under consideration to validate the developed model equation. The percentage yield of char, pyro-oil and pyrogas obtained from the validation experiments were graphically compared with the values estimated by the model equations. Also, the level of agreement of the models estimate with the experimental data was computed using Pearson correlation coefficient (Eq. (9)) to enable the assessment of the ability of the developed model to estimate products’ yield value adequately, Eq. (13) .
![]() =
=
![]() (13)
(13)
Where: ![]() - the model predicted values,
- the model predicted values, ![]() - the experimental values,
- the experimental values, ![]() and
and ![]() - the mean of the model predicted and experimental values
respectively for n - number of validation experiments.
- the mean of the model predicted and experimental values
respectively for n - number of validation experiments.
Result and Discussions
The result obtained from the proximate and ultimate analysis of the used tyre feedstock is as presented in Table 1.
Table 1. Proximate and ultimate analysis of used Michelin tyre
|
Parameters |
Values |
|
Proximate |
|
|
% Moisture |
2.67 |
|
% Volatile Matter |
71.88 |
|
% Fixed Carbon |
28.75 |
|
% Ash |
5.36 |
|
Ultimate |
|
|
% Carbon |
81.76 |
|
% Hydrogen |
6.98 |
|
% Oxygen |
3.75 |
|
% Nitrogen |
1.08 |
|
% Carbon-dioxide |
53.67 |
|
Energy (KJ/g) |
37.18 |
It compares favourably with existing values with little variations. This variation could be attributed to the fact that different types and brand of tyre has varying composition as determined by the manufacturer.
Table 2, shows the percentage yield of the pyrolysis products as obtained from the experiments using different combination of the process parameters.
Table 2. Percentage yield of used tyre pyrolysis product
|
S/N |
Process parameters |
Process outputs/Products |
|||
|
Temperature 0C |
Feedstock Size (fs) % |
Char Yield (Ychar) % |
Pyro-oil Yield (Yoil) % |
Pyro-gas Yield (Ygas) % |
|
|
1 |
400 |
0.47 |
47.3 |
43 |
9.7 |
|
2 |
400 |
1.84 |
45.8 |
45.3 |
8.4 |
|
3 |
400 |
3.28 |
44.3 |
49.9 |
5.8 |
|
4 |
400 |
0.47 |
48.3 |
41.4 |
10.3 |
|
5 |
400 |
1.84 |
46.8 |
44.3 |
8.9 |
|
6 |
400 |
3.28 |
45.3 |
48.5 |
6.2 |
|
7 |
400 |
0.47 |
47.5 |
41.7 |
10.8 |
|
8 |
400 |
1.84 |
46.3 |
44.4 |
9.3 |
|
9 |
400 |
3.28 |
44.8 |
48.5 |
6.7 |
|
10 |
500 |
0.47 |
42.6 |
44.8 |
12.6 |
|
11 |
500 |
1.84 |
39.8 |
49.3 |
10.9 |
|
12 |
500 |
3.28 |
38 |
53.1 |
8.9 |
|
13 |
500 |
0.47 |
43.6 |
43.4 |
13 |
|
14 |
500 |
1.84 |
40.8 |
47.8 |
11.4 |
|
15 |
500 |
3.28 |
38.9 |
51.7 |
9.4 |
|
16 |
500 |
0.47 |
43.1 |
43.3 |
13.6 |
|
17 |
500 |
1.84 |
40.3 |
47.7 |
12 |
|
18 |
500 |
3.28 |
38.5 |
51.6 |
9.9 |
|
19 |
600 |
0.47 |
41.2 |
36.3 |
22.5 |
|
20 |
600 |
1.84 |
39.4 |
40.1 |
20.5 |
|
21 |
600 |
3.28 |
38.4 |
42.1 |
19.5 |
|
22 |
600 |
0.47 |
42.2 |
34.8 |
23 |
|
23 |
600 |
1.84 |
40.3 |
38.7 |
21 |
|
24 |
600 |
3.28 |
39.4 |
40.1 |
19.9 |
|
25 |
600 |
0.47 |
42 |
34.5 |
23.5 |
|
26 |
600 |
1.84 |
39.9 |
38.3 |
21.8 |
|
27 |
600 |
3.28 |
38.9 |
40.6 |
20.5 |
Tables 3, 4 and 5 show the ANOVA results in respect of the effect process parameters on the percentage yield of Char, pyro-oil and pyro-gas respectively.
Table 3. ANOVA Table for char yield
|
Source |
DF |
SS |
MS |
FCalculated |
|
Temperature |
1 |
164.846 |
164.846 |
73.97 |
|
Feedstock sizes |
1 |
52.278 |
52.278 |
23.46 |
|
2-Way Interaction |
1 |
0.004 |
0.004 |
0.00 |
|
Error |
23 |
51.256 |
2.229 |
|
|
Total |
26 |
687.930 |
|
|
Tables
3-5 are each representative of the general symbolic ANOVA Table for the various
pyrolysis products. The Fcalculated which is obtained by weighting
the mean square (MS) of each source of variation against that of the
experimental error is normally used to check the effect of varying the sources
of variation on the process output. As mentioned earlier the hypothesis being
tested is whether varying the pyrolysis temperature and the feedstock size as
well as the effect of their interaction influence the process output/products
or not. Under this hypothesis, if the corresponding value of FCalculated
(column 5) is less than or equal to that of FTable (obtainable from
Table of F distribution), i.e. FCalculated ![]() FTable,
the null hypothesis that varying these factors or their interaction has no
effects on the response is true otherwise the alternate hypothesis is true and
the effect of changes in process parameters on the process response is
statistically significant.
FTable,
the null hypothesis that varying these factors or their interaction has no
effects on the response is true otherwise the alternate hypothesis is true and
the effect of changes in process parameters on the process response is
statistically significant.
From Table 3, it was observed that the Fcalculated for pyrolysis temperature (Fcalculated = 73.97) and feedstock size (Fcalculated = 23.46) in respect of char yield is greater than the corresponding FTable (i.e. F (0.05, 1, 23)) which is 4.279. Hence, Ho is rejected. Therefore the effect of change in pyrolysis temperature and feedstock sizes on the char yield obtained is statistically significant. The Table further revealed that the Fcalculated due to the interaction between the operating parameters (Fcalculated = 0.00) is less than that of the corresponding FTable. This shows that the effect of the possible interaction between the two operating parameter, as they change in value, on char yield is not statistically significant.
Table 4. ANOVA Table for pyro-oil yield
|
Source |
DF |
SS |
MS |
FCalculated |
|
Temperature |
1 |
211.692 |
211.692 |
18.66 |
|
Feedstock sizes |
1 |
215.281 |
215.281 |
18.97 |
|
2-Way Interaction |
1 |
1.567 |
1.567 |
0.14 |
|
Error |
23 |
260.956 |
11.346 |
|
|
Total |
26 |
687.930 |
|
|
Similarly, Table 4 revealed that the Fcalculated for pyrolysis temperature (Fcalculated = 18.66) and feedstock size (Fcalculated = 18.97) in respect of pyro-oil yield is greater than the corresponding FTable (i.e. F (0.05, 1, 23) = 4.279). Also, Table 5 revealed that the Fcalculated for pyrolysis temperature (Fcalculated = 204.67) and feedstock size (Fcalculated = 15.71) in respect of pyrogas yield is greater than the corresponding FTable (i.e F (0.05, 1, 23) = 4.279). This implies that the effect of change in pyrolysis temperature as well as feedstock size on pyro-oil and pyrogas yields is statistically significant.
However, the interaction of the two parameters along value change trend do not have any effect on the pyro-oil and pyrogas yields as Tables 4 and 5 shows that the Fcalculated for the 2-way interaction of the operating parameters for the two products yield is less than the FTable.
Table 5. ANOVA Table for pyro-gas yield
|
Source |
DF |
SS |
MS |
FCalculated |
|
Temperature |
1 |
747.899 |
747.899 |
204.67 |
|
Feedstock sizes |
1 |
57.393 |
57.393 |
15.71 |
|
2-Way Interaction |
1 |
0.964 |
0.964 |
0.26 |
|
Error |
23 |
84.047 |
3.654 |
|
|
Total |
26 |
891.250 |
|
|
Consequently, it is enough to infer that the percentage yield variability of the considered pyrolysis product attributable to changing the pyrolysis temperature and the feedstock size is greater than that attributable to uncontrollable experimental error. This is considered enough to conclude that varying the pyrolysis temperature and the feedstock size actually affects the yield of the pyrolysis products. Since, the interaction between the process parameters is found to have no effect on the yield the pyrolysis products considered, it is neglected for estimating the products’ yield as mentioned earlier. This is because this result reduces the third term of Eq. (8) to zero to give Eq. (9).
An evaluation of Eq. (9) using data points obtained from experiments resulted in the regression coefficients presented in Table 6.
Table 6. Regression coefficients obtained for product yield equation
|
S/N |
Product |
Yield symbol |
Regression coefficient |
||
|
β0 |
β1 |
β2 |
|||
|
1 |
Char |
Ychar |
59.73 |
-0.03039 |
-0.000452 |
|
2 |
Pyro-oil |
Yp-oil |
56.56 |
-0.03417 |
0.000917 |
|
3 |
Pyrogas |
Yp-gas |
-16.26 |
0.06450 |
-0.000473 |
When the regression coefficient β0, β1, and β2 obtained for the product yield equation in respect of Char, Pyro-oil and Pyro-gas as given on Table 6 is substituted for in Eq. (9), the result is Eqs. (10), (11) and (12) which is used for the estimation of the percentage yield of Char, Pyro-oil and pyro-gas respectively.
Figures 2-4 show the trends and relationships between the predicted and experimental value of char, pyro-oil and pyrogas yield respectively. The Figures show that the predicted values obtained from the models are in agreement with the values from experiments.
A comparison of the actual yield value of the Char, pyro-oil and pyrogas obtained from the validation experiments and the values estimated from the model equations 11, 12 and 13 revealed that the correlation coefficient is 90.00%, 78.63% and 95.11% for estimating the percentage yield of char, pyro-oil and pyrogas respectively.
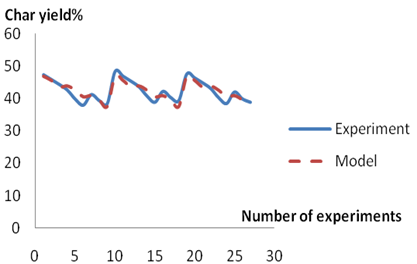
Figure 2. Comparison between experimental and model predicted values for char yield
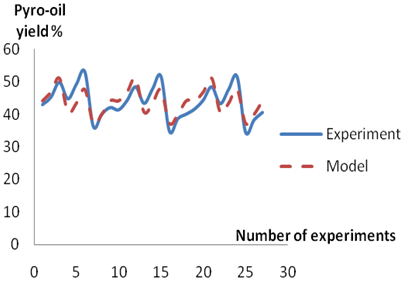
Figure 3. Comparison between
experimental and model predicted values for pyro-oil yield 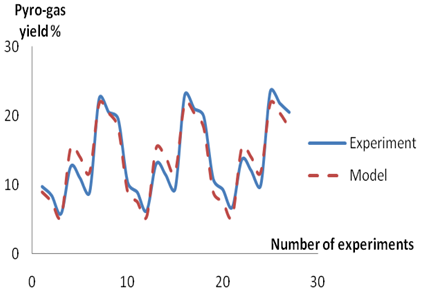
Figure 4. Comparison between experimental and model predicted values for pyro-gas yield
The model obtained for the prediction of pyrogas yield provides values that matches the experimental values relatively more than the one for predicting char which in turn is more accurate than the model for evaluating pyro-oil yield. With correlation coefficient of more than 70 % obtained in respect of the developed models, they can therefore be used as a reliable tool for determining the yield of pyrolysis products. Hence, the percentage yield of char, pyro-oil and pyrogas could be estimated from the pyrolysis temperature and feedstock size (i.e. Percentage weight) with an accuracy of 90.00 %, 78.63 % and 95.11 % respectively at 95 % confidence level.
Conclusion
Pyrolysis can be regarded as one of the techniques that aid energy recovery from solid waste. It encourages waste management and minimizes waste in our environment.
This study investigated the effect of pyrolysis temperature, feedstock size and their interaction on the yield of char, pyro-oil and pyrogas obtained from used tyre pyrolysis. It was deduced that, both process parameters each had statistically significant effect on the pyrolysis products’ yield while their interaction had no effect on the yield as the effect was found not statistically significant.
Subsequently multiple linear regression models were formulated and validated to facilitates the production of the pyrolysis products’ yield with good accuracies. The model would help in exhaustively examining the process parameters considered towards selecting the appropriate values in respect of the yield of pyrolysis being target thereby providing a means of optimizing the pyrolysis process.
References
1. Ezra B., Efim K., Roman S., Survey and examination thermal treatment of municipal solid waste and comparison of alternatives for thermal treatment of scrap tyres, Final Report, Ministry of the Environment Grant 8-300, Ben-Gurion University, Beer Sheva, Israel, 2010.
2. Ali A., Melanie L., Waste tire pyrolysis: Influential parameters and product properties, Current Sustainable/Renewable Energy Report, 2014, 1 (4), p. 129–135.
3. Pyrocrat, Industrial pyrolysis of waste plastic and tyres, 2007 (Accessed 15 October, 2016, available from: http://www.pyrolysisplant.com).
4. Jasminska N., Brestovic T., Carnogurska M., The effect of temperature pyrolysis process of used tyres on the quality of output products, Acta Mechanica et Automatica, 2013, 7 (1), p. 20-25.
5. Juma M., Korenova Z., Markos J., Annus J., and Jelemensky L., Pyrolysis and combustion of scrap tire, Petroleum and Coal, 2006, 48, p. 15-26.
6. Nhlanhla N., Edison M., A review and discussion of waste tyre pyrolysis and derived products, Proceeding of the World Congress on Engineering, 2014, 2, p. 1-7.
7. Cunliffe A.M., Williams P.T., Composition of oils derived from the batch pyrolysis of tyres, Journal of Analytical and Applied Pyrolysis, 1998, 44, p. 131-152.
8. Asraruddin G., Recycling and pyrolysis of scrap tyre, Report on training visit in the frame of project no. SAMRS 2011/06/01 funded by Slovak Aid, Bratislava, Slovakia, 2011.
9. Bridgewater A.V., Biomass fast pyrolysis, Thermal Science, 2004, 8 (2), p. 21-49.
10. Jidgarnka S., Pyrolysis of expired car tyres: Mechanics of producing high quality fuel, Chulalongkorn University, Department of Petrochemistry, 2006 (Accessed 20 January, 2017, available from: http://www.vcharkarn.com/varticle/408).
11. Williams P.T., Pyrolysis of waste tyres: a review, Waste Management, 2013, 33 (8), p. 1714 – 1728.
12. Kyari M., Cunliffe A., Williams P.T., Characterization of oils, gases, and char in relation to the pyrolysis of different brands of scrap automotive tyres, Energy and Fuels, 2005, 19 (3), p. 1165-1173.
13. Laresgoiti F.M., Chromatographic analysis of the gases obtained in tyre pyrolysis, Journal of Analytical and Applied Pyrolysis, 2000, 55, p. 43–54.
14. Debalaxmi P., Singh R.K., Thermal pyrolysis of bicycle waste tyre using batch reactor, International Journal of Chemical Engineering and Applications, 2011, 2 (5), p. 332-336.
15. Hariram V., Mohammed Ismail M.A., Design and development of pyrolysis batch reactor and characterization of tyre pyrolysis oil using GC/MS and FTIR, International Journal of Research in Engineering and Technology, 2014, 3 (11), p. 366-372.
16. Balat M., Balat M., Kırtay E. and Balat H., Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: Pyrolysis systems, Energy Conversion and Management, 2009, 50, p. 3147-3157.
17. Akinola A.O., Design of a thermochemical reactor for conversion of selected wood biomass to fuel a stationary Diesel engine, Doctoral dissertation, Mechanical Engineering Department, The Federal University of Technology, Akure, 2012.