Engineering, Environment
Neural network-based fault diagnosis in CSTR
Rishi Sarup SHARMA1*, Lillie DEWAN2 and Shantanu CHATTERJI3
1 Research Scholar, National Institute of Technology, Kurukshetra, India
2 Professor, National Institute of Technology, Kurukshetra, India
3 Director, United College of Engineering & Research, Greater Noida, India
E-mail(s): 1 rishi.sarup@gmail.com; 2 l_dewan@nitkkr.ac.in; 3 chatterjis@yahoo.com
* Corresponding author phone: 91-9896317575
Received: March 04, 2018 / Accepted: May 23, 2018 / Published: June 30, 2018
Abstract
In this paper, the experimental study has been carried out on CSTR in the presence of process faults which can occur due to sudden and unexpected change in certain process parameters. A neural network (NN) is an essential tool used in the fault diagnosis for the chemical processes, in particular. As the artificial neural networks (ANN) represent the nets of basis functions, these offer better empirical models, taking into account the complex nature of the non-linear processes. The faults like the change in flow rate and the change in agitator speed have been injected into the system simultaneously. Due to these faults, a difference in the output of CSTR, i.e. titration endpoint has been analysed. Moreover, while varying the speed of agitators, it has been observed that fault becomes prominent at high speed of each of the agitators. The experimental results have been simulated on the NN, after which the nature and magnitude of faults have been visualized.
Keywords
Continuous Stirred Tank Reactor (CSTR); Chemical Processes; Faults; Fault Diagnosis
Introduction
Control systems of today have become more complex and the control algorithms are going more sophisticated. This has witnessed a spurt in the demand for fault tolerance, which can be accomplished through the efficient fault detection, isolation and accommodation [1]. Trends in the industrial chemical processes today have incorporated automation systems with control systems for improved stability. In recent years, the increased complexity of the process plants has necessitated the use of a wide range of sensors. These sensors along with other logging devices are being employed to measure process signals. This is done in order to facilitate ease of the task of decision making for monitoring purpose [2-4]. A fault can be perceived as a kind of malfunction in a dynamic system or process plant that results in an unacceptable anomaly in the system performance [1]. Fault diagnosis entails the precise estimation of the faulty conditions captured in real time. This can thus avoid (if not completely eliminate) the occurrence of a fault or departing of process path from normal conditions. Faults can impair the system performance, degrade the product quality and can cause financial losses [5]. A system that incorporates the capability of detecting, isolating, identifying or classifying faults is termed as a fault diagnosis system. The purpose behind investigating diagnosing faults is to generate the inconsistencies among the nominal and faulty conditions. These inconsistencies arising out of the diagnosis procedure are termed as residuals. These residuals can be generated by adopting analytical methods like observers, parity equations, and parameter estimation and are based on analytical redundancy [6]. The difficulties in formulating these models are the intricacies involved in obtaining precise mathematical models as also the sensitivity associated with diagnostic system to the modelling errors [7]. In order to ensure the safety and reliability of a process, an early detection and isolation system is required [8]. Prevailing fault diagnosis approaches for chemical processes can be broadly classified into two categories: model-free techniques (based on statistical analysis, fuzzy logic, neural networks and expert systems) [9-10] and model-based techniques which rely on observer-based methods [11-15].
During the operation of a chemical process, several faults affect its safety and productivity. The fault occurrence reduces the efficiency of process (in terms of poor quality of finished product), at times posing hazard to the personnel, causing environmental pollution and damage to equipment, in certain cases. The faults that are critical in a chemical process are:
1. Actuator faults-disruption in electrical power supply, hindrance in the operation of pumps and valves.
2. Process faults- sudden and unexpected change in certain process parameters, unwanted reactions due to use of impure materials.
3. Sensor faults- sudden activation of wrong sensors indicating false alarms.
Due to the above, fault diagnosis as well as fault detection have become issues of crucial importance in the field [16].
The chemical process under study is the mixing of the two reactants namely sodium hydroxide and ethyl acetate. This is one of the important reactions taking place in the CSTR. The kinetics of reactants involves the saponification of ethyl acetate, under the alkaline conditions as per the following Eq. (1).
![]() NaOH + CH3COOC2H5
CH3COONa + C2H5OH (1)
NaOH + CH3COOC2H5
CH3COONa + C2H5OH (1)
The literature on the above saponification reaction of ethyl acetate has been focused on kinetics and reaction mechanism. Such a reaction is described as an irreversible, second order on an overall basis. But it is in fact a first order reaction, when considered with regard to its reactants. In case, equimolar concentrations of both the reactants are employed, it is observed that the behaviour of the reaction in terms of its order decreases [17].
The reactor employed in the present work is a Cascade CSTR, where continuous stirring is done to mix the two liquids with a variable flow rate. Continuous operation is the preferred mode of operation for many chemical processes. CSTR, either as a single unit (tank) or connection of multiple units in series, are mostly employed in the chemical plants. The CSTR reactor is generally employed for liquid-phase or multi-phase reactions that have fairly high reaction rates. Streams of the reactants are continuously being fed into the vessels and product streams are withdrawn. The use of CSTR is widely accepted in the organic chemical industry, chiefly due to consistent product quality, ease in control, in comparison to other reactor types [18]. The CSTR may be operated in steady state, implying thereby that no variables vary with time. Here the ideal conditions are termed to be that in which the flow rates are assumed to be constant [19].
The objective of the paper lies in determining the faulty conditions, encountered during the process dynamics in CSTR. The operating conditions in the CSTR include the alkaline hydrolysis of ethyl acetate, in the presence of sodium hydroxide. This is also referred to as saponification. It is defined as the hydrolysis of a fat or oil (in the form of alkali) to yield soap for cleaning and reaction purpose. It refers to the process of hydrolysis of an ester under alkaline conditions to produce alcohol along with salt of a carboxylic acid (in the form of carboxylates). The sodium hydroxide, present in this reaction, acts as a caustic base. The vegetable oils and animal fats, present in the form of esters, act as triglycerides. When an alkali reacts with an ester, it breaks the bonds within the esters and yields a fatty acid salt along with glycerol [20]. An attempt to determine the mathematical models for non-linear processes has been the issue of prime importance today. In a plant, which bears a non-linear relationship between the different dependent and independent variables, it is difficult to accurately design an effective controller. The ANN possess a remarkable capability of approximating a non-linear relationship with arbitrary reasonable accuracy, if suitable architecture and weight parameters are properly chosen. ANN have been used in a variety of engineering applications, as they act as mathematical aids in mathematical modelling, apart from other applications of pattern recognition and system identification. An amazing feature of ANN is the learning capability from past experiences or data, adapting to new changes and generalizing which coincides with the humans. It can extract features from the historic training data by making use of a suitable training algorithm, thus obviating the need of a priori knowledge of process. This helps a non-linear system to be modelled with enhanced flexibility. Parameter estimation is required in cases where either the data is not known exactly or is not known at all. This job can be efficiently handled by using the ANN which helps in fault diagnosis. The diagnosis part checks whether the norm of parameter change is greater than a predefined threshold [21-22].
The ANN can be conveniently applied in the domain of fault diagnosis. This can be done through model approximation and pattern recognition. Pattern recognition has become an easy approach in the solution of fault diagnosis problems, as it aids in the determination of size of fault. It is most often an off-line procedure, where readings for normal and fault situation can be simulated through training. Thereby, it can establish whether the situation is normal or faulty. Recently, the potential of neural network for fault diagnosis has been demonstrated. NN based approach is especially suitable when accurate mathematical models are too difficult to be generated for some processes. NN attempt to mimic the computational structures of the human brain [21, 23-24]. A NN is employed for learning the relationship between symptoms and faults, extracted from the process data. After training, such relationships are stored in the form of network weights. This network, so formed, is subsequently trained to classify the symptoms into corresponding faults. The NN can have inputs in the form of qualitative values or on-line measurements, taken from the process. In case, the on-line values act as network inputs, quite a good number of training sets are required to simulating the neural network [25]. A MATLAB program for simulating the experimental readings in the neural network has been made that takes into account the different targets and inputs and then subsequently produces the outputs.

Figure 1. Experimental set-up for CSTR
Material and method
This section underlines the importance of the procedure adopted to obtain the readings employed in the experiment, so as to achieve the fault plots.
(i) Initially a pre-determined amount of solution from the agitator 3 (Figure 1) is taken in a beaker. The solution is the end-result of the mixing of the reactants sodium hydroxide and ethyl acetate mix in the two tanks (Figure 2) in the requisite concentration (Table 2). This reading is said to be taken from the reactor when a small amount of solution from agitator 3 (Figure 1) is poured into the beaker.
(ii) Then a small amount of phenolphthalein indicator is poured into the beaker in the solution obtained from the step (i) above.
(iii) Then there is topping up of sodium hydroxide from the burette required to fill into the beaker for reaching the titration end point. The titration end point is reached when the presence of phenolphthalein indicator in the beaker after mixing with the sodium hydroxide in the burette turns the solution (obtained from the step (ii) above) into light pink colour.
(iv) The amount of sodium hydroxide required to reach titration end point is measured in terms of millilitres from the gradations of the different markings given on the outer surface of the beaker. This is termed as the output of the chemical reactor (CSTR).
(v) The faults then correspond to the difference in the values obtained under nominal and fault conditions.
(vi) The faults generated experimentally are then simulated on a neural network, by way of MATLAB program. Different variations are then introduced in the program to inculcate the fault conditions and subsequent output is then taken and compared with experimental output.
The methodology adopted for the fault analysis in CSTR is quite simple. The titration end point achieved as an end result is taken to be the output of the CSTR system. However, the reactor operates under both nominal and fault conditions, In order to evaluate the performance of CSTR, the readings are first experimentally taken under nominal conditions when the ideal conditions exist. Under the effect of faults, the system behaves in a different manner. The NN simulates the CSTR output, by way of MATLAB program. This network output is then compared with the experimental output, in order to ascertain the error between the experimental and neural network simulation results. Under the various fault conditions duly specified in the tables [6 to 11] that follow, the network returns different outputs.
Experimental study of CSTR
The process under study is a Cascade CSTR is as shown in Figure 1, where continuous stirring has been done to mix the two liquids with a variable flow rate.
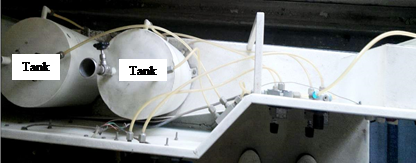
Figure 2. Tanks containing the reactants and connected in cascade
Continuous operation is the preferred mode of operation for many chemical processes. Streams of the reactants are continuously fed into the vessels and product streams are withdrawn. The cascade CSTR employs mixing of reactants ethyl acetate and sodium hydroxide using phenolphthalein as indicator, which was contained in the tanks shown in Figure 2.
The solution of ethyl acetate and sodium hydroxide was first prepared and put in the tanks 1 and 2 respectively. After this, both these solutions get mixed with each other and the resultant solution flows in the first vessel (corresponding to agitator 1), as shown in Figure 1. This solution further passes on to the next subsequent vessel and resultant solution comes out of the last and final vessel (corresponding to agitator 3). With each successive observation obtained, the volumes in both the tanks keep decreasing.
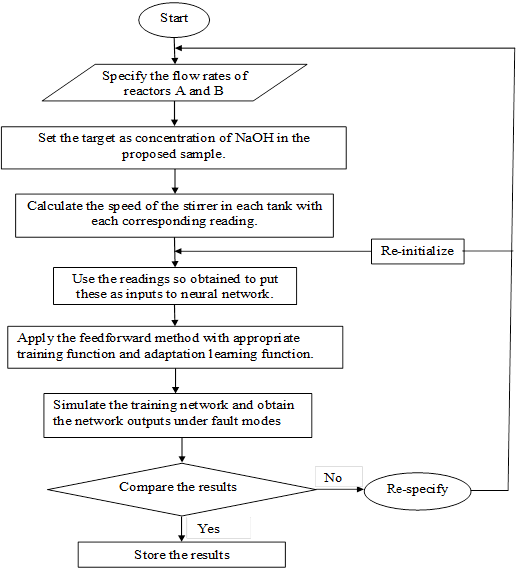
Figure 3. Flowchart for simulation of experimental results in Neural Network
The following flowchart (Figure 3) has been formulated in order to illustrate the actual steps that go into visualizing the performance of the CSTR using neural networks.
The problem
The CSTR is an interesting problem that has been taken up by the researchers all through the past so many years. Each problem has been viewed from a different perspective and also presented, keeping in mind a distinct objective [13]. In the present work, the chemical reactor (CSTR) has been taken up with a view to study the different faults. It is hazardous to study the reactor, while in operation. Hence the faults are injected under laboratory conditions to study the behaviour of CSTR. In the experimental set-up shown above (figure1), there are multi-inputs (2 flow rates of the reactants viz. ethyl acetate and sodium hydroxide and 3 agitator speeds corresponding to each of the agitators). The observations achieved under the fault conditions open up new directions for research.
The proposed solution
As it is not possible to formulate a model based on the analytical redundancy, hence for process models exhibiting non-linear behaviour (for CSTR described as above), neural networks come as a handy tool to mimic the non-linear behaviour, exhibited by the chemical reactor. In the present work, the neural networks are employed for simulating the process behaviour. The experimentation has been done on CSTR, by injecting the faults (by varying process parameters). The injected faults occur in the form of variation in flow rates of the respective reactants and speeds of the corresponding agitators. As a consequence, a change in the experimental output is observed. The outputs obtained from the neural networks are found to be consistent with those acquired through experimentation. The model is then compared with the results achieved through the experiment performed under laboratory conditions. The residuals, so formulated, act as a basis of the conclusions drawn thereafter. This is outline of the work presented. The main contributions of the paper are enumerated as:
(i) The faults are modelled through the neural network, since for making the models of the process the use of analytical equations becomes difficult.
(ii) The residuals are described in terms of numerical values, so that correct comparisons can then be made. The residuals are being generated using neural networks as well as experimental results.
(iii) The magnitude of the residuals describes the fault severity. The higher the values, the severe are the faults. Both the NN values and the experimental values act as fault indicators for expressing the fault situation.
(iv) From the above, fault severity gives a guideline as to which faults are more severe, and correspondingly under which conditions. From the numerical values, the fault severity can be predicted using neural network model as well experimental readings.
(v) From the Tables (from 6 to 11), there is another revelation: in most of the cases, the neural network prediction errors for the various faults are quite less (numerically) than those, given through the experimental observations.
The novel aspects of the work are outlined above for easy reference. Although there are different areas in which the neural networks have been applied, yet the prominent papers in which the neural networks have been employed are the following:
1. Fault detection in a robotic manipulator – corresponding to [26].
Here in the above paper, the residual analysis is done for the robotic manipulator arm using hybrid neural networks. The results are shown in terms of plots depicting nominal and faulty conditions. However, in the present paper, the numerical deviations are calculated through the neural network model and subsequently a comparison is made with regard to the same with the experimental analysis. Hence this can lead to better judgement as compared to the above paper.
2. Polymer quality prediction using neural networks – corresponding to [27]
The quality of the product has been monitored in the laboratory under off-line conditions. The results of the experimental data were made available after several hours for each of the iteration. But in the present paper, the data has been obtained in the laboratory conditions after obtaining the titration end point which also took several minutes under varying conditions of the faults injected. So after a several hundred readings for the injected faults, the neural network was then implemented through the MATLAB program, which gave the desired results.
3. Neural-Networks-based Fault Detection and Accommodation – corresponding to [28]
In the above paper, the experimental work is done and the faults are being plotted against the fault-free values. But the overall picture is grim, as no direct conclusions can be drawn from the represented in the tabular form. Nevertheless, the present paper clearly mentions the depiction and deviation for the normal values. This way, the judging becomes easy and one can easily make out the whole situation. The tabular results convey both the deviations, in terms of percentage, for the experimental analysis and well as the neural networks. Hence the demarcation line between the nominal and faulty operations gets endowed with clarity.
4. Modelling of CSTR using neural networks – corresponding to [29]
Here the steady state conditions of the CSTR are discussed and then the same conditions are mapped onto the neural network. The results are shown in terms of errors from plant data and neural networks. But the results of the present paper are modelled using program and do not make use of the toolbox. In the paper, the architecture of the neural network is described in terms of the parameters that are easily available in the toolbox
5. Fault detection and diagnosis for CSTR using NN – corresponding to [30].
Here the conditions and governing equations of the CSTR are clearly stated. The results are expressed in terms of error plots with indications for each of the faults. But here, the problem is that the manner, in which the errors are calculated, is not explicitly stated. Contrary to this, the present paper clearly mentions how the errors are computed on the basis of the mean values. And interestingly, the tabular form also takes into account, the percentage basis and not the difference values, as the quantitative measure, which is more meaningful.
Proposed algorithm for Artificial Neural Network (ANN)
The observations taken during the course of experimentation is taken into account. During the experiment, the variations are done in the following: speed of agitators and flow rates of the respective reactants viz. ethyl acetate and sodium hydroxide. The outputs achieved under these conditions of variation are used inputs. This is a multi-input and single-output (MISO) system. Following steps are taken to develop the source code for ANN in MATLAB:
a. Input the values – flow rates of the two reactants, the speeds of the three agitators under nominal conditions.
b. Input the conditional values- flow rates and agitators speeds under varying fault conditions, as enumerated in Table 4 below.
c. Form the feed forward networks with training algorithms corresponding to the fault and nominal conditions.
d. Outputs are taken in the form of arrays and then compared with those obtained under experimental conditions.
The interconnection of the different volumes from each of the vessels as indicated in Figure 1 can be represented as a block diagram depicted in Figure 4.
![]()
Ca0 Ca1 Ca2 Ca3
Figure 4. Block diagram of CSTR
Here the output of each preceding vessel acts as an input to the succeeding one.
Where: V1, V2, V3 are the volumes of the vessels and Ca1, Ca2, Ca3 are the concentration of the reactants.
The governing equations pertaining to the dynamic variations occurring in the amounts of reactants, for each of the three tanks [31] are given below, Eq. (2-4):
![]() (2)
(2)
![]() (3)
(3)
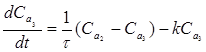 (4)
(4)
The Figure 5 gives the process flow from the start, where the mixing of the two reagents ethyl acetate and sodium hydroxide takes place onto the first vessel.
Y0 V1, k1 V2, k2 V3, k3
Y1 Y2 Y3
Figure 5. Flow diagram of the different process variables in CSTR
This continues up to the next vessel and further to the last and final vessel from where the mixture drains out. Here k1, k2 and k3 correspond to the reaction rates of the different stages. The small letter k pertains to the gain. Y0 corresponds to the input and Y1, Y2 and Y3 represent the subsequent outputs of the each of the successive stages.
Governing equations for flow of volume
The governing equation for the volume flow through the tanks is given below, Eq. (5):
Volume in the Tank = Input volume - Output volume - Volume due to reaction (5)
Mathematically, this implies the following Eq. (6-8)
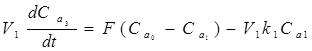 (6)
(6)
![]() (7)
(7)
![]() (8)
(8)
Where: V1, V2, V3 - volumes of the vessels in which the agitators are running (m3), Ca0, Ca1, Ca2, Ca3 - initial concentrations of the reactants (kmol/m3), k1, k2, k3 - reaction rates under different stages (min-1), dCa3/dt - time rate of change of concentration inside the tank, kmol/m3/sec.
Reaction rate is given by Arrhenius equation kn = αe-E/ RTn
Where: n = 1, 2, 3…., denotes the stage number, as indicated by Eq. (4), (5) and (6) [31-32].
Parameters, concentration and constants of different components of chemical reactor used in the experiment are as given in Table 1, 2 and 3 respectively.
Table 1. Nominal parameters and their specific values
|
S.No. |
Parameter |
Value |
|
1. |
Reaction constant (τ) |
0.2 |
|
2. |
Gain (k) |
0.5 |
|
3. |
Step size delta |
0.1 |
Table 2. Concentration of reactors
|
S.No. |
Reactor |
Concentration (kmol/m3) |
|
1. |
Initial Ca0 |
1.8 |
|
2. |
Ca1 |
0.4 |
|
3. |
Ca2 |
0.2 |
|
4. |
Ca3 |
0.1 |
Table 3. Working parameters of the CSTR
|
S.No. |
Parameter of tank |
Value |
|
1. |
Height of the tank |
200 mm |
|
2. |
Inside Diameter |
140 mm |
|
3. |
Volume of Tank |
3.078 Litres |
|
4. |
Height of Liquid in the tank |
160mm |
|
5. |
Working volume of tank |
2.46 litres |
|
6. |
Agitation speed (variable) |
0-350 rpm |
|
7. |
Fluid used |
Ethyl acetate, sodium hydroxide |
|
8. |
Fluid flow measurement |
0-19 litres per hour |
Experimental observations and discussions
Under the normal condition of chemical reactor, the flow rates of sodium hydroxide and ethyl acetate and the speed of agitators should be the same. Variation in either flow rate or agitator speed results in change in the output which can be referred to as fault present in the system. To monitor the behaviour of the system in the presence of faults i.e. intentional faults were introduced in the CSTR available in the laboratory.
Experimental observations were taken under the following conditions:-
Case I: When no fault is present in the system which corresponds to Table 4.
Case II: When flow rate of sodium hydroxide / ethyl acetate is varying, governed by the flow meter FA and FB respectively and alongside the speed of any one of the agitators speed is varied while the other two agitators remain at constant speed. These are summarized in Table 5, given below.
Table 4. Observations taken under ideal conditions
|
Sr. No. |
Flow Rate A |
Flow Rate B |
Time Elapsed |
ON |
|
1 |
5 |
5 |
300 |
25.6 |
|
2 |
5 |
5 |
500 |
25.8 |
|
3 |
5 |
5 |
700 |
26.0 |
|
4 |
5 |
5 |
900 |
25.9 |
|
5 |
5 |
5 |
1100 |
26.1 |
|
6 |
8 |
8 |
1300 |
26.0 |
|
7 |
8 |
8 |
1500 |
25.9 |
|
8 |
8 |
8 |
1700 |
25.8 |
|
9 |
8 |
8 |
1900 |
25.7 |
|
10 |
8 |
8 |
2100 |
25.9 |
|
11 |
11 |
11 |
2300 |
25.8 |
|
12 |
11 |
11 |
2500 |
26.0 |
|
13 |
11 |
11 |
2700 |
26.0 |
|
14 |
11 |
11 |
2900 |
26.1 |
|
15 |
11 |
11 |
3100 |
26.2 |
|
16 |
15 |
15 |
3300 |
26.0 |
|
17 |
15 |
15 |
3500 |
25.9 |
|
18 |
15 |
15 |
3700 |
25.7 |
|
19 |
15 |
15 |
3900 |
25.5 |
|
20 |
15 |
15 |
4100 |
25.6 |
|
21 |
19 |
19 |
4300 |
25.8 |
|
22 |
19 |
19 |
4500 |
25.7 |
|
23 |
19 |
19 |
4700 |
25.9 |
|
24 |
19 |
19 |
4900 |
26.0 |
|
25 |
19 |
19 |
5100 |
26.1 |
ON - Output at normal speed
Table 5. Summary of intentional faults
|
Injected Fault |
Description |
|
F1 |
Change in flow rate of ethyl acetate solution and agitator speed of Stirrer 3 low with stirrer 2 and 1 at normal |
|
F2 |
Change in flow rate of ethyl acetate solution and agitator speed of Stirrer 3 medium with stirrer 2 and 1 at normal |
|
F3 |
Change in flow rate of ethyl acetate solution and agitator speed of Stirrer 3 high with stirrer 2 and 1 at normal |
|
F4 |
Change in flow rate of sodium hydroxide solution and agitator speed of Stirrer 3 low with stirrer 2 and 1 at normal |
|
F5 |
Change in flow rate of sodium hydroxide solution and agitator speed of Stirrer 3 medium with stirrer 2 and 1 at normal |
|
F6 |
Change in flow rate of sodium hydroxide solution and agitator speed of Stirrer 3 high with stirrer 2 and 1 at normal |
|
F7 |
Change in flow rate of ethyl acetate solution and agitator speed of Stirrer 1 low with stirrer 2 and 3 at normal |
|
F8 |
Change in flow rate of ethyl acetate solution and agitator speed of Stirrer 1 medium with stirrer 2 and 3 at normal |
|
F9 |
Change in flow rate of ethyl acetate solution and agitator speed of Stirrer 1 high with stirrer 2 and 3 at normal |
|
F10 |
Change in flow rate of sodium hydroxide solution and agitator speed of Stirrer 1 low with stirrer 2 and 3 at normal |
|
F11 |
Change in flow rate of sodium hydroxide solution and agitator speed of Stirrer 1 medium with stirrer 2 and 3 at normal |
|
F12 |
Change in flow rate of sodium hydroxide solution and agitator speed of Stirrer 1 high with stirrer 2 and 3 at normal |
|
F13 |
Change in flow rate of ethyl acetate solution and agitator speed of Stirrer 2 low with stirrer 1 and 3 at normal |
|
F14 |
Change in flow rate of ethyl acetate solution and agitator speed of Stirrer 2 medium with stirrer 1 and 3 at normal |
|
F15 |
Change in flow rate of ethyl acetate solution and agitator speed of Stirrer 2 high with stirrer 1 and 3 at normal |
|
F16 |
Change in flow rate of sodium hydroxide solution and agitator speed of Stirrer 2 low with stirrer 1 and 3 at normal |
|
F17 |
Change in flow rate of sodium hydroxide solution and agitator speed of Stirrer 2 medium with stirrer 1 and 3 at normal |
|
F18 |
Change in flow rate of sodium hydroxide solution and agitator speed of Stirrer 2 high with stirrer 1 and 3 at normal |
The last column pertains to the output and is measured in millilitres (ml.).
Case II – In this case, the speeds of all the agitators are varied one by one, in turn, while the fault is introduced by varying the flow rates of both the flow meters A and B, as depicted in figure 1. Mentioned below are the sub-cases for each of the agitators 1, 2, and 3 (as depicted in Figure 1).
1. Fault in flow rate A and B and fault due to variation in speed of stirrer 3
Table 6. Faults and their simulated parameters under various operating conditions
|
S. No. |
Process variable |
Fault Type |
Relation of FA and FB |
Observation |
Agitator 1 Speed |
Agitator 2 Speed |
Agitator 3 Speed |
Mean Output Value (Neural Network) |
Mean Output Value (Experimental) |
% Change Simulated Neural Output |
% Change Experimental Output |
|
1. |
Flow Rate |
No fault |
Equal |
Output Unchanged |
Normal |
Normal |
Normal |
25.9 |
- |
- |
- |
|
2. |
Flow rate |
F1 |
FB changed FA constant |
Variation in output |
Normal |
Normal |
Low |
26.28 |
22.2 |
1.467 |
-14.285 |
|
3. |
Flow rate |
F2 |
FB changed FA constant |
Variation in output |
Normal |
Normal |
Medium |
26.43 |
29.0 |
2.046 |
11.969 |
|
4. |
Flow rate |
F3 |
FB changed FA constant |
Variation in output |
Normal |
Normal |
High |
25.68 |
29.9 |
-0.849 |
15.444 |
Fault Conditions: Change in flow rate B in agitator 3
Table 7. Faults and their associated parameters under various operating conditions
|
S. No. |
Process variable |
Fault Type |
Relation of FA and FB |
Observation |
Agitator 1 Speed |
Agitator 2 Speed |
Agitator 3 Speed |
Mean Output Value (Neural Network) |
Mean Output Value (Experimental) |
% Change Simulated Neural Output |
% Change Experimental Output |
|
1. |
Flow Rate |
No fault |
Equal |
Output Unchanged |
Normal |
Normal |
Normal |
25.9 |
|
- |
- |
|
2. |
Flow rate |
F4 |
FA changed FB constant |
Variation in output |
Normal |
Normal |
Low |
25.65 |
24.2 |
-0.965 |
-6.563 |
|
3. |
Flow rate |
F5 |
FA changed FB constant |
Variation in output |
Normal |
Normal |
Medium |
25.73 |
31.2 |
-0.656 |
20.463 |
|
4. |
Flow rate |
F6 |
FA changed FB constant |
Variation in output |
Normal |
Normal |
High |
26.08 |
31.6 |
0.694 |
22.008 |
Fault Conditions: Change in flow rate B in agitator 3.
2. Fault in flow rate A and B and fault due to variation in speed of stirrer 1
(i) Fault Conditions: Flow rate B changed as fault in agitator 1
Table 6 shows that the variation brought about by a change in flow rate of flow meter B, containing sodium hydroxide solution, leading to fault F6 is most prominent as compared to all other fault. Table 7 enlists the fault initiated by variation in flow rate A. However, the fault lowest magnitude is of fault F4 and F2 though both these faults have emanated from the same cause i.e. variation in flow rates of the flow meter A and B, which is quantified in Table 6. But of these two, the least magnitude is of F4 which is due to change in flow rate of flow meter A, containing sodium hydroxide solution. The flow rates in tank 1, having ethyl acetate and 2 having sodium hydroxide, are varied from 60% to 380%. When there is slow drift in the reaction kinetics, due to variation in flow rates, it leads to drifting of reactor output from their normal steady-state values. Table 6 and 7 enlists the operating conditions linked with these.
Table 8. Faults and their associated parameters under various operating conditions
|
S. No. |
Process variable |
Fault Type |
Relation of FA and FB |
Observation |
Agitator 1 Speed |
Agitator 2 Speed |
Agitator 3 Speed |
Mean output value (NN) |
Mean output value (experimental) |
% Change simulated neural output |
% Change experimental output |
|
1. |
Flow Rate |
No fault |
Equal |
Output Unchanged |
Normal |
Normal |
Normal |
25.9 |
|
- |
- |
|
2. |
Flow rate |
F7 |
FB changed FA constant |
Variation in output |
Low |
Normal |
Normal |
25.83 |
23.4 |
-0.27 |
-9.652 |
|
3. |
Flow rate |
F8 |
FB changed FA constant |
Variation in output |
Medium |
Normal |
Normal |
25.87 |
31.7 |
-0.116 |
22.394 |
|
4. |
Flow rate |
F9 |
FB changed FA constant |
Variation in output |
High |
Normal |
Normal |
26.12 |
33.4 |
0.849 |
28.957 |
(ii) Fault Conditions: Flow rate A changed as fault in agitator 1
In Table 9, the faults are depicted to have the highest magnitude though in both the cases, the flow rate of flow meter A, containing sodium hydroxide solution is varied.
Table 9. Faults and their associated parameters under various operating conditions
|
S. No. |
Process variable |
Fault Type |
Relation of FA and FB |
Observation |
Agitator 1 Speed |
Agitator 2 Speed |
Agitator 3 Speed |
Mean output value (NN) |
Mean output value (experimental) |
% Change simulated neural output |
% Change experimental output |
|
1. |
Flow Rate |
No fault |
Equal |
Output Unchanged |
Normal |
Normal |
Normal |
25.9 |
|
- |
- |
|
2. |
Flow rate |
F10 |
FA changed FB constant |
Variation in output |
Low |
Normal |
Normal |
24.95 |
26.0 |
-3.67 |
0.386 |
|
3. |
Flow rate |
F11 |
FA changed FB constant |
Variation in output |
Medium |
Normal |
Normal |
24.83 |
33.5 |
-4.13 |
29.343 |
|
4. |
Flow rate |
F12 |
FA changed FB constant |
Variation in output |
High |
Normal |
Normal |
25.902 |
33.9 |
0.008 |
30.888 |
Fault Conditions: Flow rate A changed as fault in agitator 1.
However, there is one striking difference: the speed of agitator 1 is varied from medium to high. The fault with highest magnitude is F12 which corresponds to the low speed of agitator 1. Table 8 describes the faults injected by variation in the flow rate of B, by way of changing the speed of agitator 1. It is observed that fault F7 has a downward trend and interestingly this too corresponds to the low speed of agitator 1. Table 9 enlists the operating conditions associated with this sub-case. Table 8 depicts the F9 as the highest deviation in terms of fault accruing from experimental readings. Whereas the errors in case of agitator 1 at high speed, are comparable under variation in flow meter and that of flow meter B.
3. Fault in flow rate A and B and fault due to variation in speed of agitator 2
(i) Fault Conditions: Flow rate B changed in agitator 2.
In the Table 10 above, the faults are depicted to have the relatively less magnitude then given in Table 9, though in both the cases, the flow rate of flow meter B, containing ethyl acetate solution is varied.
Table 10. Faults and their associated parameters under various operating conditions
|
S. No. |
Process variable |
Fault Type |
Relation of FA and FB |
Observation |
Agitator 1 Speed |
Agitator 2 Speed |
Agitator 3 Speed |
Average Output Value (Neural Network) |
Mean Output Value (Experimental) |
% Change Simulated Neural Output |
% Change Experimental Output |
|
1. |
Flow Rate |
No fault |
Equal |
Output Unchanged |
Normal |
Normal |
Normal |
25.9 |
|
- |
- |
|
2. |
Flow rate |
F13 |
FB changed FA constant |
Variation in output |
Normal |
Low |
Normal |
25.81 |
21.8 |
-0.347 |
-15.830 |
|
3. |
Flow rate |
F14 |
FB changed FA constant |
Variation in output |
Normal |
Medium |
Normal |
26.42 |
28.8 |
2.01 |
11.197 |
|
4. |
Flow rate |
F15 |
FB changed FA constant |
Variation in output |
Normal |
High |
Normal |
26.33 |
28.8 |
1.66 |
11.197 |
(ii) Fault Conditions: Flow rate A changed in agitator 2
The fault with lowest magnitude is F13 which corresponds to the low speed of agitator 2. Table 10 describes the faults injected by variation in the flow rate of B, by way of changing the speed of agitator 2. It is observed that while the agitator 2 speed goes from medium to high, there is no change, as far as the errors arising out of experimental outputs are concerned. Table 10 enlists the operating conditions associated with this sub-case.
Table 11. Faults and their associated parameters under various operating conditions
|
S. No. |
Process variable |
Fault Type |
Relation of FA and FB |
Observation |
Agitator 1 Speed |
Agitator 2 Speed |
Agitator 3 Speed |
Mean Output Value (Neural Network) |
Mean Output Value (Experimental) |
% Change Simulated Neural Output |
% Change Experimental Output |
|
1. |
Flow Rate |
No fault |
Equal |
Output Unchanged |
Normal |
Normal |
Normal |
25.9 |
- |
- |
- |
|
2. |
Flow rate |
F16 |
FA changed FB constant |
Variation in output |
Normal |
Low |
Normal |
26.09 |
21.1 |
7.33 |
-18.533 |
|
3. |
Flow rate |
F17 |
FA changed FB constant |
Variation in output |
Normal |
Medium |
Normal |
25.77 |
31.1 |
-0.502 |
20.077 |
|
4. |
Flow rate |
F18 |
FA changed FB constant |
Variation in output |
Normal |
High |
Normal |
25.996 |
32.1 |
0.37 |
23.938 |
In the Table 11, the fault F16 has been shown to have the highest magnitude which corresponds to the variation in the flow rate of sodium hydroxide solution and high speed of agitator 2. Another peculiar case is that of downward or negative trend depicted in fault F17 which corresponds to the low speed agitator 2 but variation in output is more in case the flow rate of meter A, containing sodium hydroxide solution, is varied. Table 11 lists the various operating conditions for the faults associated with sub-section 3.
Significant Contributions of the Manuscript
(i) The neural network technique has been implemented on the process modelling of CSTR, which is an MISO (multi-input, single-output) in nature.
(ii) The use of neural network has demonstrated that the percentage error has been reduced, in comparison to the experimental error, existing between the nominal and injected fault values.
(iii) A relative comparison has been made vis-a-vis the different operating fault conditions, on the basis of which it becomes easy to gauge the severity of the fault injected in CSTR.
(iv) This analysis can serve as a guide to alert the operator during the course of operation in case of CSTR. It helps to aid the operator in decision-making process for dealing with CSTR.
The above findings are significant in the field of process modelling and analysis for CSTR.
Conclusion
The paper offers the experimental procedure for the chemical reactor (in this case, the CSTR), adopted for the purpose of fault diagnosis. This experimental set-up investigates the practical aspects of a practical industrial process. The experiment explores the feasibility of identifying faults and the desired results have been obtained in the process simulation of CSTR. Through the plots obtained from the experimental results of CSTR, the simulation and generation of faults has been depicted and the relative magnitude of these faults is shown in figure 8. As there are 5 inputs (2 flow rates of the reactants and 3 agitator speeds) and one output (achieved in the form of titration end point), it essentially becomes a multi-input and single-output (MISO) system. As the process monitoring and modelling can be achieved efficiently though neural network, hence this approach has been applied here. The results, as stated in the introduction above, coincide with those of the experimental observations (taken under nominal and varying fault conditions). This is evident from the Table 6 to Table 11 above. In the tables, the neural network outperforms the experimental data in representing the MISO system of the CSTR above. The present work is targeted towards providing useful information which supports the decision-making of the operator, dealing with the chemical reactor.
References
1. Frank P.M., Fault diagnosis in dynamic systems using analytical and knowledge-based redundancy - A survey and some new results, Journal of Automatica, 1990, 26 (3), p. 459-474.
2. Gomero F.I., Melendez J., Colomer J., Process diagnosis based on qualitative trend similarities using a sequence matching algorithm, Journal of Process Control, 2014, 24, p. 1412-1424.
3. Zhang Z., Dong F., Fault detection and diagnosis for missing data systems with three-time slice dynamic Bayesian network approach, Journal of Chemometrics and Intelligent Laboratory Systems, 2014, 138, p. 30-40.
4. Yan B., Wang H., Wang H., A novel approach to fault diagnosis for time-delay systems, Journal of Computers and Electrical Eng., 2014, 40, p. 2273-2284.
5. Marseguerra M., Zio E., Monte Carlo simulation for model-based fault diagnosis in dynamic systems, Journal of Reliability Engineering and System Safety, 2009, 94, p. 180-186.
6. Calado J.M.F., Korbicz J., Patan K, Paton R.J., Sa Da Costa, J.M.G, Soft computing approaches to fault diagnosis for dynamic systems, European Journal of Control, 2001, 7, p. 248-286.
7. Lo C.H., Wong Y.K., Rad A.B., Model-based fault diagnosis in continuous dynamic systems, ISA Transactions, 2004, 43. p. 459-475.
8. Isermann R., Ball P., Trends in the application of model-based fault detection and diagnosis of technical processes, Journal of Control Engineering Practice, 1997, 5 (5), p. 709-719.
9. Isermann R., Model-based fault detection and diagnosis - Status and applications, Annual Reviews in Control, 2005, 29 (1), p. 71-85.
10. Caccavale F., Pieeri F., Iamarino M., Tufano V., An integrated approach to fault diagnosis for a class of chemical batch processes, Journal of Process Control, 2009, 19, p. 827-841.
11. Gertler J. Designing dynamic consistency relations for fault detection and isolation, International Journal of Control, 2000, 73 (8), p. 720-732.
12. Venkatasubrmanian V., Rengaswamy R., Yin K., Kavuri S.N., A review of process fault detection and diagnosis - Part I: Quantitative model-based methods, Journal of Computers and Chemical Engineering, 2003, 27, p. 293-311.
13. Venkatasubrmanian V., Rengaswamy R., Yin K., Kavuri S.N., A review of process fault detection and diagnosis -Part III: Process History-based Methods, Journal of Computers and Chemical Engineering, 2003, 27, p. 327-346.
14. Ali J.M., Hoang N.H., Hussain M.A., Dochain D., Review and classification of recent observers applied in chemical process systems, Computers and Chemical Engineering, 2015, 76, p. 27-41.
15. Zhao J., Wei H., Guo W., Zhang K., Singularities in the identification of dynamic systems, Journal of Neuro-Computing, 2014, 104, p. 339-344.
16. Gertler J., Fault detection and isolation using parity relations, Journal of Control Engineering Practice, 1997, 5 (5), p. 653-661.
17. Ahmad A., Muhammad I.A., Muhammad Y., Khan H., Shah M.H., A comparative study of alkaline hydrolysis of ethyl acetate using design of experiments, Iranian Journal of Chemistry and Chemical Engineering, 2013, 32 (4), p. 33-47.
18. Danish M., Mesfer M.K.A., Rashid M.M., Effect of operating conditions on CSTR performance: an experimental study, International Journal of Engineering Research and Applications, 2015, 5 (2), p. 74-78.
19. Schmidt L.D., The engineering of chemical reactions, Second Edition. London: Oxford University Press, 2005, p. 51-52.
20. Ullah I., Ahmed M.I., Optimization of saponification reaction in continuous stirred tank reactor, NFC-IEFR Journal of Engineering and Scientific Research, 2013, 1, p. 73-77.
21. Azhar S., Rehman S.A.B., Application of neural network in fault detection study of batch esterification process, International Journal of Engineering & Technology IJET-IJENS, 2011, 10 (3), p. 45-51.
22. Patan K., Artificial neural network for the modelling and fault diagnosis of technical processes, Springer-Verlag (First Edition), 2008.
23. Lipnickas A., Two-stage neural network based classifier system for fault diagnosis. In: computational intelligence in fault diagnosis, Springer-Verlag, 2006.
24. Simani S., Model-based fault diagnosis in dynamic systems using identification techniques, PhD Thesis. Dottarato Di Ricerca, 2003, p. 20-27
25. Zhang J, Morris J., Process modelling and fault diagnosis using neural networks, Journal of Fuzzy Sets and Systems, 1996, 79, p. 127-140.
26. Anand D.M., Selvraj T., Kumanan S., Fault detection and isolation in robotic manipulator via hybrid neural networks, International Journal of Modelling and Simulation, 2008, 7, p. 5-16.
27. Himmelblau D.M., Applications of artificial neural networks in chemical engineering, Korean Journal of Chemical Engineering 2000, 17 (4), p. 373-392.
28. Saludes S., Fuente M.J., Neural-networks-based fault detection and accommodation in a chemical reactor, Proceedings of 14th Triennial World Congress of IFAC, 1999, p. 7849-7854.
29. Suja Malar R.M., Thyagrajan T., Modelling of continuous tank reactor using artificial intelligence techniques, International Journal of Modelling and Simulation, 2009, 8 (3), p. 145-155.
30. Rahman R.Z.A., Soh A.C., Muhammad N.F.B., Fault detection and diagnosis for continuous stirred tank reactor using neural network, Kathmandu University Journal of Science, Engineering and Technology, 2010, 6 (2), p. 66-74.
31. Luyben W.S., Process modelling, simulation and control for chemical engineers, Tata Mc Graw Hill International Edition (2nd Edition), 1996.
32. Sharma R.S., Dewan L., Chatterji S., Experimental analysis of process faults of CSTR, Journal of Electrical Engineering Romania, 2017, 17, p. 375-383.