Engineering, Environment
Water absorption characteristics of Epoxy matrix composites reinforced with green silica particles
Oluyemi Ojo DARAMOLA1, Olajide Samson AKINTAYO2
1-2 Department of Metallurgical and Materials Engineering, Federal University of Technology, Akure, Ondo State Nigeria
E-mail(s): 1 oodaramola@futa.edu.ng; 2 akintayoolajide@yahoo.com
* Corresponding author, phone: +2348166814002
Received: May 19, 2018 / Accepted: June 27, 2018 / Published: June 30, 2018
Abstract
Water uptake characteristics of epoxy matrix composites reinforced with green silica particles extracted from rice husk ash have been investigated. The composites was developed by incorporating the rice husk silica particles in the weight fractions of 0.5, 1, 2, 3, 4 and 6% in an epoxy resin using manual mixing and hand layup method. Water immersion test was used to evaluate the water absorption behaviour from which the water diffusion mechanisms of the developed composites were established. Results show that the water uptake process appears to be diffusion controlled. The weight gained and percentage water absorption by all the composites increases with immersion time and increasing particle loading respectively. The highly filled samples showed higher water absorption which is largely due to agglomeration of rice husk silica particles. Parameters such as saturated moisture content, diffusion coefficient and sorption coefficient are dependent on filler content. The diffusion curve fitting analysis which evaluates the diffusion exponent (n) values show that all the composites developed exhibited Less Fickian behaviour (also considered as Fickian diffusion mechanism). This implies that the water penetration rate is very much below the polymer chain relaxation rate.
Keywords
Epoxy resin, Rice husk silica, Fickian diffusion, Water absorption, Sorption
Introduction
Polymer Matrix composites (PMCs) are manufactured commercially for many diverse applications such as sporting goods, aerospace industry, vehicle structural applications, industrial heavy duty floorings [1,2]. PMCs possess good flexibility, corrosion resistance, specific strength, ductility and process ability. However, their major limitation is water absorption which is very pronounced in marine and humid environments Water molecules can diffuse into the network of composites to affect the mechanical properties. Polymer matrix composites differ from other materials in the sense that low- molecular weight substances such as water may easily migrate into them even at room temperature resulting in a variation of the material's structure, properties, morphology, and composition [3]. This phenomenon takes place either in the matrix or at the filler/matrix interface since water cannot penetrate the filler. In some cases, it could lead to an irreversible degradation of the material in the so called humid aging that includes both chemical aging and physical aging [4]. The epoxy matrix show moisture sensitivity due to interactions between some polar groups of the macromolecule and the water molecules, which leads to a reduction of both glass transition (Tg) and mechanical properties.
This sensitivity increases with the increasing degree of cross - linking and also with the polarity concentration of the molecular groups [5]. The prediction of water diffusion, the mechanism and its influence on the matrix properties are essential to estimate long term behaviour and service life of polymer matrix composites. Moisture uptake in PMCs usually is measured by weight gain and the water diffusion mechanism is characterized by Fick’s law of diffusion [6]. The physical phenomena that characterizes moisture uptake are dissolution, diffusion, swelling, and relaxation, together with deformation and stress build up in the matrix [7]. Water absorption behaviour of polymer composites decreases its mechanical and dimensional properties as matrix cracking and particle debonding can also modify the mechanisms of water penetration in the composite thereby providing new routes for moisture ingress [8, 9]. Hence, the need to study the water absorption behaviour of particulate reinforced composites is not far-fetched as it could give rise to the potential use of these composites in outdoor applications and humid environmental conditions. The present study investigates the water absorption characteristics of epoxy matrix composites incorporated with varying weight fraction of rice husk silica particles. In addition, we also studied the water diffusion phenomena of the developed composites.
Material and Method
Rice husk silica particles which was extracted from rice husk was used for this work. The rice husk was collected from a rice processing mill at Army Barracks along Ondo road, Akure, South-West, Nigeria. The Epoxy resin system along with its curing hardener (SL 1000 grade) was obtained from a chemical store in Lagos state, Nigeria while the reagents (hydrochloric acid, sodium hydroxide) and ash less filter paper were purchased from Pascal Chemical Ltd, Akure, Ondo state, Nigeria.
Combustion of rice husk into rice husk ash (RHA)
First, the rice husk (RH) was thoroughly washed with water and dried. The dried rice husk was fed into an enclosed drum and burnt into ash. This burnt ash was conditioned in a muffle furnace at a temperature of 650oc for three hours to obtain a grey rice husk ash (see Fig. 1a).
Figure 1. (a) The conditioned rice husk ash (b) Rice husk silica
Silica powder extraction
The silica gel was extracted from RHA according to Daramola et al. [10]. 80g of rice husk ash (RHA) was added to sodium hydroxide (NaOH) solution with a concentration and volume of 2.5 M and 990 ml respectively in a beaker.
The beaker containing the mixture was placed in a water shaker bath and heated at 1000C for 1 hour. The solution was then allowed to cool to room temperature and then filtered through a Whatman no. 42 ash less filter paper. The filtrate and the carbon residue were both collected in separate containers.
Concentrated hydrochloric acid was then added to the obtained filtrate and then stirred continuously. The PH of the solution was checked until a value of 7.0 was reached to allow precipitation of silica gel. Ageing was done for 48 hours to promote silica gel formation. The silica gel produced was separated from the soluble solution with the aid of the vacuum filtration pump. The silica gel was dried in an air blast oven for 48 hours. The obtained white silica gel was then pulverized into silica powder. The pulverized silica powder was then subjected to sieving process to obtain the required particle size as shown in Figure 1(b).
Chemical composition and particle size analysis of the rice husk silica (RHS) particles
The extraction yield and purification parameter of rice husk silica from RHA was estimated using the Energy Dispersive X-ray (EDX) spectrometer. The particle size of the rice husk silica powder was analysed using Horiba dynamic light scattering (DLS) particle size analyser.
The measurable particle size range of the instrument is 0.05-3000 μm and it is equipped with a small volume sample dispersion unit. About 0.5 g of the silica powder was dispersed in deionised water in the sample dispersion unit of the instrument, vigorously mixed for about two minutes at speed of 2100 rpm, and sonicated for 45 seconds. The ultrasonic waves were used to break or minimise any particle agglomerates that may be present in the suspension. Measurements were taken and the diffraction data and graphs recorded by the instrument’s software program.
Composite production
Manual mixing method and hand lay-up (open moulding technique) were used for the composite production. The production was carried out at room temperature. The composite samples were prepared using the 0.5, 1, 2, 3, 4 and 6 wt. % fractions of the rice husk silica powder. The matrix material (epoxy resin and curing hardener) was prepared in the ratio of 2 parts of epoxy resin to 1 part of the curing hardener (2:1). The silica powder was first added to the epoxy resin and mixed thoroughly before adding the hardener.
All forms of weight measurements were carried using an electronic weighing balance. The mixture was stirred to ensure homogeneity and poured into an aluminium mould pre-coated with a lubricant to aid easy removal of the cured samples. For neat epoxy cast sample, only the epoxy and hardener was mixed and poured into the mould. Levelling was done to uniformly fill the mould cavity. The mixture was then left at room temperature to cure for two hours before removal of the composite samples. Representative composite samples utilized for the water immersion test is shown in Figure 2.

Figure 2. Representative composite samples utilized for the water immersion test
Sample designation
The composites produced were given certain designations depending on the content of the rice husk silica particles incorporated. The neat epoxy was denoted as EPS0. The designations EPS1, EPS2, EPS3, EPS4, EPS5 and EPS6 were associated with the composites reinforced with 0.5,1, 2, 3, 4 and 6 wt. % of rice husk silica particles respectively.
Water absorption test
The water absorption tests were carried out following the recommendations specified in ASTM D5229M-12 [11]. Each composite sample was dried in an oven to remove surface moisture before weighing. The weight of the oven dried samples was reported as the initial weight of the composites. The samples were then placed in distilled water maintained at room temperature (250C); and at time intervals of 24 hours, the composite samples were removed from the water, cleaned using a dry cloth and weighed. The weight measurements were taken periodically at time intervals of 24 hours for up to 168 hours. This was after water saturation in all the composite samples had been noticed. The percentage water absorbed by the composites was calculated using the Eq. (1).
![]() (1)
(1)
Where: W (%) - percentage water absorption, W0 and Wt are the oven dry weight, and the weight of the sample after time t, respectively.
Graphical plots of weight gained–immersion time and percentage water absorption plot for all the composites samples produced was made. The water absorption curve obtained for all the composite samples was used for analysis of the diffusion mechanism and kinetics. Composites usually conform to Fickian diffusion profiles, but this fact is verified with the experimental data of the different composite compositions. The mechanism of water diffusion into the composites was studied by analysing the slope and intercepts of the water absorption graphs plotted by using the relations in Eq. (2) and (3) according to [12]. The experimental data were fitted to a logarithmic equation (Eq. 3) whose slope (n) helps determines the diffusion case.
![]() (2)
(2)
![]() (3)
(3)
Where: MT is water absorption at interval
time T which is the time of immersion, ![]() is
water absorption at saturation point or mass of water absorbed at equilibrium, k is a constant parameter related to the polymer network
structure and n is the diffusion exponent value that determined the type of
diffusion mechanism.
is
water absorption at saturation point or mass of water absorbed at equilibrium, k is a constant parameter related to the polymer network
structure and n is the diffusion exponent value that determined the type of
diffusion mechanism.
The diffusion, sorption and permeation coefficients were studied using the Fickian model [13]. The diffusion coefficient or diffusivity D was calculated by the following correlation, Eq. (4):
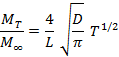 (4)
(4)
Where: L is the sample thickness and T is immersion time. The sorption coefficient S can be calculated according to Eq. (5).
![]() (5)
(5)
Where: ![]() is the initial mass of the composite sample.
is the initial mass of the composite sample.
The permeation coefficient P is also calculated thus, Eq. (6):
![]() (6)
(6)
Where: D is the diffusion coefficient and S is the sorption coefficient.
For convenience, a graphical flowchart of the entire experimental procedure utilized in this work is presented in Figure 3.
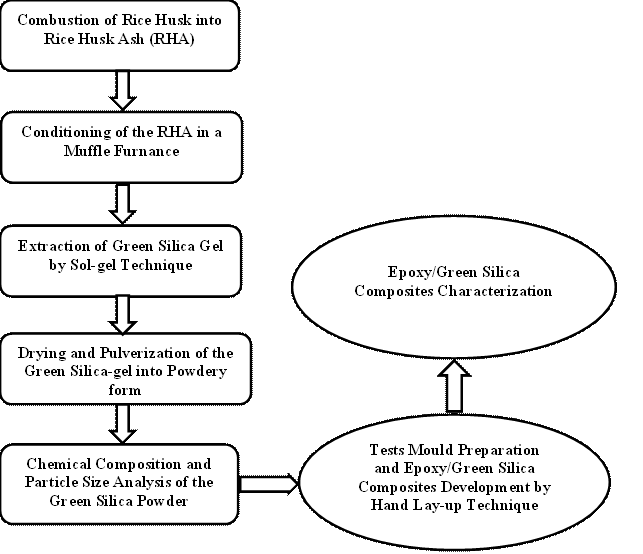
Figure 3. Graphical flowchart of the experimental Procedure
Results and Discussions
Chemical composition of the silica gel
To evaluate the effectiveness of the purification parameter and to confirm the presence of silica, EDX analysis was carried out on the silica powder extracted from RHA at 2.5 M concentration of Sodium Hydroxide (NaOH) according to Daramola et al. [10]. The major elements present are silicon and oxygen with other impurities such as carbon, phosphorus, chlorine, sodium and potassium.
Figure 4 shows the EDX elemental spectra of the silica powder extracted from RHA with 2.5 M NaOH concentration, which contains 65.35 wt % Si and 30.93 wt % O. The silicon and oxygen peaks that were observed confirm the presence of silica. The EDX spectrum shows that the content of Si and O is 96.28 in wt % and 97.80 in atomic %.
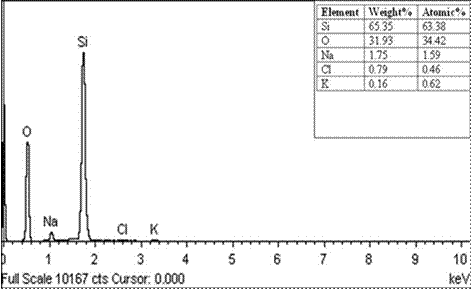
Figure 4. EDX spectrometric data of silica extracted from RHA with 2.5 M NaOH
Particle size analysis
The chemical composition of silica powder extracted from RHA has been shown to be mainly composed of silica (SiO2). The particle size of filler materials usually has a remarkable influence on the properties of composite produced. Hence, the cumulative particle size distribution of the silica powder extracted is shown in Figure 5. The particle size distribution of the silica powder is found to be approximately 0.50 µm.
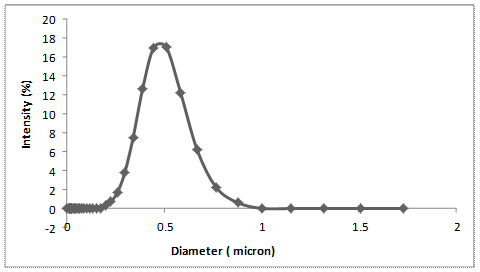
Figure 5. The particle size distribution of the rice husk silica powder (RHS) with average particle size of 0.5 μm
Water absorption behaviour of the composites
The water absorption curve for the different composite samples showing a plot of the weight gained-immersion time is presented in Figure 6.
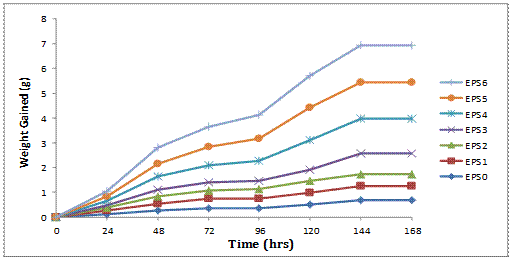
Figure 6. Graphical plot of weight gained against immersion time for the composites
Water absorption in composite materials assesses the increase in weight of the composite samples for different immersion times. It can be seen from the plot that the water absorption by the composites increases monotonically with immersion time until equilibrium condition is reached after 168 hrs at which saturation was experienced in all the samples. The characteristics of Fickian behaviour is that the absorption curve should be linear initially and the moisture content should reach saturation level at large values of immersion time. It can also be observed that an increase in content of silica powder produces a corresponding increase in water absorption for the same values of immersion time. The hygroscopic expansion in composites is affected by properties of resin such as the monomer, the polymerization rates, the cross-linking and pore size of the polymer network, the bond strength, the interaction between polymer and water, the filler and the resin-filler interface [14].
Table 1. Saturated moisture content, calculated diffusion coefficient, sorption coefficient and permeation coefficient of the composites
|
Composition |
Designation |
(%) |
Dx10 -9 (m2/s) |
S (g/g) |
Px10 -10 (m2/s) |
|
Neat Epoxy |
EPS0 |
2.97 |
7.56 |
1.02 |
77.1 |
|
0.5 wt% SiO2 |
EPS1 |
2.86 |
6.04 |
1.03 |
62.2 |
|
1.0 wt% SiO2 |
EPS2 |
3.11 |
3.19 |
1.03 |
32.9 |
|
2.0 wt% SiO2 |
EPS3 |
5.02 |
4.24 |
1.05 |
44.5 |
|
3.0 wt% SiO2 |
EPS4 |
6.61 |
7.35 |
1.06 |
78.0 |
|
4.0 wt% SiO2 |
EPS5 |
6.81 |
7.94 |
1.07 |
85.0 |
|
6.0 wt% SiO2 |
EPS6 |
7.13 |
7.15 |
1.08 |
77.2 |
Some important parameters can be determined
from the water absorption curve in Figure 7. The first parameter is known as
the saturation water absorption of the composites (![]() ), usually referred to as equilibrium value for the water
content when the samples are exposed to immersion for a long period of time.
Some observations can be made from the saturation parameter results shown in
Table 1.The saturated water content (
), usually referred to as equilibrium value for the water
content when the samples are exposed to immersion for a long period of time.
Some observations can be made from the saturation parameter results shown in
Table 1.The saturated water content (![]() ) depends on the rice husk silica content of the
composites. As the silica content is increasing, the saturated water content (
) depends on the rice husk silica content of the
composites. As the silica content is increasing, the saturated water content (![]() ) also experiences a corresponding increase.
) also experiences a corresponding increase.
Another vital parameter of Fick’s model that can be determined is the diffusion coefficient (D) which shows the ability of the water molecules to penetrate inside the composite structure. The diffusion coefficient (𝐷) of the composites was calculated by the measurements of weight gain and the initial slope of the weight gain curves versus square root of time according to [15]
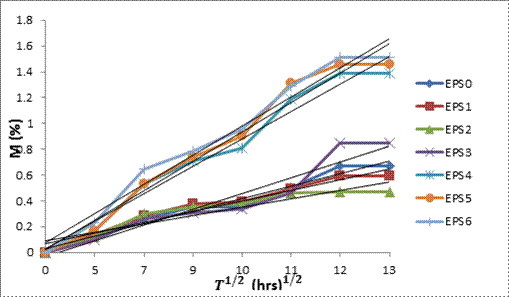
Figure 7. Water absorption curves for the composites
The values of the diffusion coefficients are presented in Table 1 above. The diffusion coefficient of the neat epoxy (EPS0) is higher than a good number of the composite samples. This is because of reaction between epoxy and water molecules through the matrix/filler interface, thus making a separation between the epoxy and rice husk silica particles [16]. Previous study has shown that diffusion coefficient of particulate epoxy composites is lesser than the neat epoxy resin [17]. Sorption is the absorption ability of water into materials. The value of the sorption coefficients of the composites is also presented in Table 1. It can be seen that the sorption coefficients increases linearly with increase in filler content implying that sorption depends on the filler content. This is in agreement with the Kittikorn et al. [13]. The increase in sorption coefficient is quite small and approximately equal to one for all samples. Permeation is defined as the penetration of water through a material as calculated from equation (6). As observed from the data in Table 1, the permeation of water through the composites with higher silica content is large compared those with lower silica content.
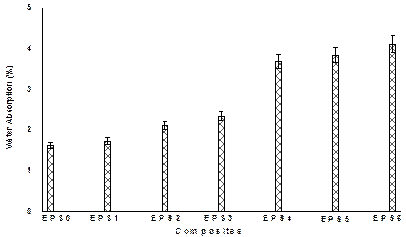
Figure 8. Percentage water absorption of the samples
Figure 8 revealed that percentage water absorption increases as the particle loading increases. The data indicates that water absorption for the composites is higher than for the neat epoxy. The increase in silica content increases the volume of interfacial zone where water molecules can be easily trapped. Moreover, composites with higher silica concentration possess high moisture absorption which is attributed to the agglomeration experienced by silica particles at higher loading [18].
Water diffusion mechanism in the composites
Figure 9 shows a plot of Log ![]() versus Log T for the composites produced which gives the
values of slope (n) used in establishing the mechanism of water diffusion. The
prediction of water diffusion and its influence on the matrix properties are
essential to estimate long term behaviour and service life of composites.
Moisture uptake in PMCs usually is measured by weight gain and the water
diffusion mechanism is characterized by Fick’s law of diffusion [6]. The
phenomenon of water penetration into polymer matrix composites (PMCs) consists
of diffusion of water molecules into the micro-voids between polymer chains.
Other common mechanisms includes capillary transport into the gaps and flaws at
the matrix/filler interface, incomplete wettability and impregnation of filler
in matrix and transport by micro-cracks in the matrix formed during processing
[6,19].
versus Log T for the composites produced which gives the
values of slope (n) used in establishing the mechanism of water diffusion. The
prediction of water diffusion and its influence on the matrix properties are
essential to estimate long term behaviour and service life of composites.
Moisture uptake in PMCs usually is measured by weight gain and the water
diffusion mechanism is characterized by Fick’s law of diffusion [6]. The
phenomenon of water penetration into polymer matrix composites (PMCs) consists
of diffusion of water molecules into the micro-voids between polymer chains.
Other common mechanisms includes capillary transport into the gaps and flaws at
the matrix/filler interface, incomplete wettability and impregnation of filler
in matrix and transport by micro-cracks in the matrix formed during processing
[6,19].
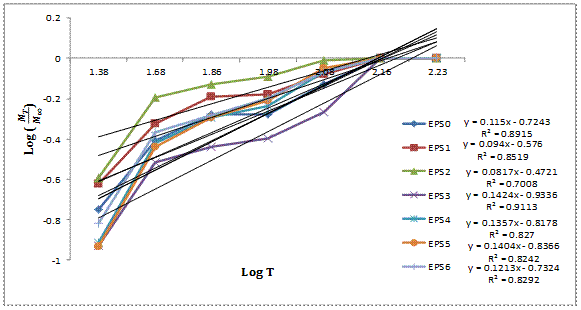
Figure 9. Diffusion curve fitting plot for the composites
Water diffusion mechanism in PMCs can be categorized according to the relative mobility of the penetrant (water molecules) and of the polymer segments (monomers). Due to these factors, three categories of diffusion mechanisms have been established according to Shen and Springer [6]. If the rate of penetrant (water molecules) diffusion is much less than that of the polymer segment mobility, Fickian diffusion (Case I) is dominant. For this mechanism, the diffusion exponent (n) is 0.5. The saturation inside the polymer composite is quickly reached and it is maintained with independence of time [20]. If the rate of penetrant (water molecules) mobility is much greater than other relaxation processes (polymer segment mobility), Case II prevails. The value of n is 1.0 and indicates that diffusion process is more rapid than the relaxation process. This diffusion mechanism is characterised by the development of a boundary between the swollen outer part and the inner glassy core of the polymer matrix. The boundary advances at a constant velocity and the core size reduces until an equilibrium penetrant concentration (saturation) is reached in the entire polymer matrix composite [21]. For Non-Fickian (or anomalous) diffusion, the diffusion exponent is an intermediate value (0.5 < n < 1). This mechanism indicates situations when the penetrant mobility and the polymer segment relaxation are equally matched.
The anomalous behaviour is an intermediate between the Fickian case and Case II diffusion mechanisms [22]. There are cases where n >1, such as usually referred to as Super Case II mechanism [23]. However, if the water penetration rate is very much below the polymer chain (monomer) relaxation rate, it is possible to record the n values below 0.5. This scenario, which is classified also as Fickian diffusion, is called as ‘Less Fickian’ behaviour [24, 25]. These three cases of diffusion can be distinguished theoretically by the shape of the water absorption curve represented by power law expression given by Sombastsompop and Chaochanchikul [12]. Upon fitting the experimental data into equation (3), the plot in Figure 8 is presented. It is observed from figure 8 that all the composites exhibited the shapes of straight line graphs which are obtained by curve fitting in agreement with equation (3).
Table 2. Water sorption constants of the composites
|
Sample |
Slope (n) |
Intercept (k) |
R2 |
|
EPS0 |
0.8150 |
0.7243 |
0.8915 |
|
EPS1 |
0.0940 |
0.5760 |
0.8519 |
|
EPS2 |
0.0817 |
0.4721 |
0.7008 |
|
EPS3 |
0.1424 |
0.9336 |
0.9113 |
|
EPS4 |
0.1357 |
0.8178 |
0.8270 |
|
EPS5 |
0.1404 |
0.8366 |
0.8242 |
|
EPS6 |
0.1213 |
0.7324 |
0.8292 |
The water sorption constants n and k calculated from the fitting of the experimental data to equation (3) have been presented in Table 2.
Table 3. Water diffusion mechanism for the produced composites
|
Type of diffusion mechanism |
Slope (n) |
Time dependence |
Composites |
Range of water absorption |
|
Less Fickian
|
n < 0.5 |
t-1/2 |
EPS1,EPS2,EPS3, EPS4,EPS5,EPS6 |
1.73-4.09 |
|
Fickian Diffusion |
n = 0.5 |
t1/2 |
- |
- |
|
Non-Fickian (Anomalous) Diffusion |
0.5 < n < 1.0 |
tn-1 |
EPS0 |
0-1.62 |
|
Case II Diffusion |
n = 1.0 |
Time independent |
- |
- |
|
Super Case II Diffusion |
n > 1.0 |
tn-1 |
- |
- |
A summary of the water diffusion mechanisms in the composites produced is also presented in Table 3. It can be observed from Table 2 that the diffusion exponent (n) value ranges between 0.0817-0.8150. For neat epoxy, the diffusion coefficient value (0.815) is in the range of 0.5<n<1.0 which indicates that is exhibits Non-Fickian (anomalous) diffusion where the diffusion rate and the relaxation chain are equally matched. However, all the composite samples produced exhibited Less Fickian diffusion behaviour since their diffusion exponent (n) values are far lesser than 0.5. This implies that the water penetration rate is very much below the polymer chain (monomer) relaxation rate.
This behaviour can also be classified as Fickian diffusion mechanism [24, 25]. None of the composite samples developed exhibited Case II or Super Case II diffusion. It is also established that for the neat epoxy that possess lower water absorption, the diffusion exponent is higher compared to the composites which possess increasing water absorption and lesser diffusion exponent values.
Conclusion
In this work, the water absorption characteristics of epoxy matrix composites reinforced with varying weight fractions of rice husk silica particles have been investigated. Submicron silica particles (0.50 µm) was successfully extracted from rice husk ash (RHA) using sol-gel process.
The silica content in the particle was found to be 96.28 wt. %. The silica particles extracted was used to produce epoxy-green silica composites. Results revealed that the neat epoxy would perform better than the green silica composites in applications that requires long-term exposure to water or humid conditions as the silica addition led to increase in water absorption in the composites. Amongst the composites, those with lower silica content (especially EPS1-0.5wt% SiO2) can be considered for these applications. At higher loading, the silica addition increases the volume of interfacial regions which traps water, hence, highly filled composites should not be utilized for long-term water exposure and humid applications.
Also, results revealed that incorporation of green silica particles led to a change from a Non-Fickian diffusion process in the neat epoxy matrix to a Less Fickian diffusion process in all the developed composites. This implies that the water penetration rate is way lesser than the polymer chain (monomer) relaxation rate. Conclusively, all composites samples exhibited Fickian diffusion behaviour.
References
1. Njuguna J., Francesco S. and Sophia S., Nanocomposites for vehicle structural applications, Nanofibers-production, properties and functional applications, Dr. Tong Lin (Ed.), InTech., 2011.
2. Alavi Nikje M.M., Khanmohammadi M.R., Garmarudi A.B. and Haghshenas M., Nanosilica reinforced epoxy floor coating composites: preparation and thermophysical characterization, Current Chemistry Letters 1, 2012, p. 3-20.
3. Fuller R.T., Fornes R.E. and Memory S.D., NMR study of water absorbed by epoxy resin, Journal of Applied Polymer Science, 1979, 23, p. 1871-1874.
4. Sheelan R.A., The study of solution absorption and diffusion coefficient in Epoxy composite reinforced with glass fibers, Engineering and Technology, 2008, 26 (10), p. 1235.
5. Apicella A., Tessiri R. and Decataldis C., Sorption modes of water in glassy epoxies, J. memb. Sci. 1984, 18, p. 211-215.
6. Shen C. and Springer S.G., Moisture absorption and desorption of composite materials, Journal of Composite Materials, 1976, 10, p. 2-20.
7. Van krevelen D.W., Properties of polymers, Elsevier, 1997, Amsterdam.
8. Deng H., Reynolds C.T., Cabrera N.O., Barkoula N.M., Alcock B. and Pejis T., The water absorption behaviour of all-polypropylene composites and its effect on mechanical properties, Composites: Part B, 2010, 41, p. 268–275.
9. Kushwaha P.K. and Kumar R., Studies on water absorption of bamboo‐epoxy composites: Effect of silane treatment of mercerized bamboo, Journal of Applied Polymer Science, 2010, 115 (3), p. 1846-1852.
10. Daramola O.O., Oladele I.O. and Adewuyi B.O., Preparation of submicron silica gel from rice husk ash using water shaker bath, Acta Technica Corviniensis - Bulletin of Engineering, 2015, 3, p. 61-64.
11. American Society of Testing and Materials, Standard test method for moisture absorption property and equilibrium condition of polymer matrix composite materials, ASTM D5229M-12, ASTM, 2012.
12. Sombastsompop N., Chaochanchaikul K., Effect of moisture content on mechanical properties, Thermal and structural stability and extruded texture of Poly (vinyl chloride)/Wood Sawdust Composites, Polymer International, 2004, 53, p. 1210 – 1218.
13. Kittikorn T., Emma S., Monica E. and Karlsson S., Comparison of water uptake as function of surface modification of empty fruit bunch oil palm fibres in PP bio composites, surface modified composite, Bio Resources, 2013, 8 (2), p. 2998-3016.
14. Mohamad J.S., Izran K., Mechanical and physical properties of low density Kenaf core particle boards bonded with different resins, Journal of Science and Technology, 2011, p. 17-32.
15. Muńoz E., García-Manrique J.A., Water absorption behaviour and its effect on the mechanical properties of flax fibre reinforced bioepoxy composites, International Journal of Polymer Science, Hindawi Publishing Corporation, 2015, http://dx.doi.org/10.1155/2015/390275
16. Mayers M.E. and Abu- Isq I.A., Effect of water absorption on impact strength of epoxy-glass fibres composite, J. Appl. Polym. Sci., 1986, 32, p. 2515-2520.
17. Asmaashawky K., The effect of particles as additives on water absorption for epoxy resin, International Journal of Application or Innovation in Engineering & Management (IJAIEM), 2013, 2 (5), p. 131-136.
18. Narjes I.K., Water absorption and mechanical properties of high–density polyethylene /Ferric chloride composite, International Journal of Advanced Research in Chemical Science (IJARCS), 2015, 2 (8), p. 19-23.
19. Lin Q., Zhou X. and Dai G., Effect of hydrothermal environment on moisture absorption and mechanical properties of wood floor - filled polypropylene composites, Journal of Applied Polymer Science, 2002, 85 (14), p. 2824-2832.
20. Thwe M.M. and Liao K., Effects of environmental ageing on the mechanical properties of Bamboo-Glass fiber reinforced polymer matrix hybrid composites, Composites Part A, 2002, p. 43 – 52.
21. Liu W., Mohanty A.K., Drazil L.T., Mistra M., Kurian J.V. and Miller R.W., Injection moulded glass fiber reinforced poly (Trimethylene Terephthalate) composites fabrication and properties evaluation, Industrial Engineering Chem. Research, 2005, 44, p. 857- 19.
22. Khare A.R. and Peppas N.A. Swelling/Deswelling of anionic copolymer gels, Biomaterials, 1995, 16, p. 559-567.
23. Munday D.L. and Cox P.L., Compressed Xanthan and Karaya gum matrices: hydration, erosion and drug release mechanisms, International Journal of Pharmacy, 2002, 203, p. 179 – 192.
24. Wang J., Wu W. and Lin Z., Kinetics and thermodynamics of the water sorption of hydroxyethyl methacrylate/styrene copolymer hydrogels, Journal of Applied Polymer Science, 2008, 109, p. 3018 – 3023.
25. Ganji F., Vasheghani-Farahani S. and Vasheghani-Farahani E., Theoretical description of hydrogel swelling: A review, Iran Polym. Journal, 2010, 19, p. 375-398.