Engineering, Environment
Coagulation-Flocculation process of Citropsis articulata seed powders as natural coagulant for textile effluent
Kovo Godfrey AKPOMIE 1,*, Francis Kolawole OJO 2, Timothy Marhiere AKPOMIE 3, Mark Agaba ABUH 4
1Department of Pure & Industrial Chemistry, University of Nigeria, Nsukka, Nigeria
2Department of Chemical Sciences, Bingham University, Karu, Nigeria
3Department of Chemistry, Federal University Lafia, Lafia, Nigeria
4Projects Development Institute (PRODA,) Federal Ministry of Science and Technology, Emene, Enugu State, Nigeria
E-mails: kovo.akpomie@unn.edu.ng; fktdavid@yahoo.com; akpomiet@yahoo.com; markabuh1@gmail.com
*Corresponding author, phone: +2348037617494)
Received: December 28, 2017 / Accepted: June 26, 2018 / Published: June 30, 2018
Abstract
This study evaluated the potential of Citropsis articulata seed powders (CASP) for the removal of turbidity and chemical Oxygen Demand (COD) from textile effluent through coagulation-flocculation mechanism. The textile effluent collected was characterized to determine the pH, temperature, Electrical Conductivity (EC), turbidity, Dissolved Oxygen (DO), Biochemical Oxygen Demand (BOD), COD, Total Dissolved Solids (TDS), Total Hardness (TH), Total Alkalinity (TA), chloride, sulphate, calcium, magnesium and the presence of heavy metal ions. The results showed high levels of pollution of the effluents of all parameters analysed except pH, temperature and chloride exceeding the maximum permissible limit recommended by the World Health Organization. A turbidity of 254.7NTU and COD of 1402.7mg/L was obtained for the textile effluent. The effect of CASP dosage, flocculation time and temperature all showed an increase in percentage reduction of turbidity and COD with increase in these factors in the coagulation-flocculation experiment. The highest turbidity and COD reduction were 59.2% and 48.3% respectively at optimum CASP dosage of 10g/L, flocculation time 120min and temperature 320K. The result of this study indicated that CASP could be utilized as a natural coagulant in the reduction of turbidity and COD in high turbid wastewaters but not when high removal efficiencies are needed in such operations.
Keywords
Coagulation; Citropsis articulata; Effluent; Flocculation; Turbidity
Introduction
Increasing population of humans around the globe and the growth of science and technology has resulted in the establishment of many textile industries. A textile dye effluent usually contains strong colour, high total dissolved solids, high chemical oxygen demand and turbidity. The most polluted wastewaters are usually generated from the dye-houses of textile industries [1]. The textile industry is facing a great challenge in the disposal and treatment of wastewaters generated. Often, effluents from various industries have high levels of turbidity and needs to be treated by coagulation-flocculation processes in order to reduce the turbidity [2]. A lot of coagulants and flocculants have been widely applied in the treatment of industrial effluents. The materials are mainly classified into synthetic organic polymers (such as polyacrylamide derivate and polyethylene imine), inorganic coagulants (aluminium and iron based) and hybrid materials [3]. These three materials are very effective in the removal of turbidity from wastewaters [4]. Coagulation-flocculation is one of the most important treatment steps in wastewater treatment plants. Coagulation is the destabilization of particles in effluent and tends to overcome the factors that promote particles stability resulting in the formation of agglomerates or flocs [3]. Flocculation on the other hand is the process whereby destabilized particles are induced to come together forming larger agglomerates [5]. To achieve a desired level of water quality, the coast and availability of coagulating agents plays a major role. Synthetic organic polymers and aluminium salts are commonly used but these coagulants are often expensive which hinders their use in developing countries like Nigeria in the treatment of industrial effluents to minimize cost. Also, the high sensitivity of inorganic coagulants to water pH and the possibility of secondary contamination of receiving water bodies with traces of toxic synthetic polymeric coagulants or residual iron and aluminium ions are the main disadvantages of using these coagulants [6]. The residual aluminium ions in treated waters have been reported to be responsible for Alzheimer’s disease [7]. Furthermore, due to the non-biodegradability of synthetic polymers, the sludge formed in water treatment plants during flocculation-coagulation has limited potential for recycling [8]. Therefore, low cost coagulants with efficient and biodegradable potentials are required to be applied in coagulation-flocculation processes. This has led to the search and growing interest in the use of natural and food grade coagulants from renewable and relatively low cost materials [9]. The natural coagulants which are mainly polysaccharides and proteins are considered eco-friendly in comparison with inorganic and organic coagulants because of their accessibility and biodegradability [10]. The use of various natural coagulants for efficient treatment of water and wastewater has been widely reported recently [11]. Some of the natural coagulants that have been utilized include; Hibiscus esculentus seed pods [8], Moringa oleifera seed [12], plantain peel ash extract [13], Cactus latifera and seed powder of Prosopis juliflora [14], Plantago ovata extract [9], Surjana seed, maize seed and chitosan [2], chestnut and acorn [15] and Cassia angustifolia seed gum [16].
However, despite the abundance of Citropsis articulata in West Africa and Nigeria in particular, a thorough literature search revealed a lack of information on the use as a coagulant for wastewaters. Citropsis articulata was chosen due to its abundance and can be utilized as a low cost biodegradable coagulant for treatment of contaminated effluents in the available region if found to be effective. In this regard, this study reports for the first time the use of Citropsis articulate seed powder as natural coagulant in the reduction of turbidity of polluted water. The aim of this research was to investigate the potential of Citropsis articulata seed powder as a natural low cost bio-coagulant for textile effluent.
Materials and Methods
Preparation of natural coagulant
Citropsis articulata fruits were purchased from Ogbete, market, Enugu State, Nigeria. The fruits were sliced opened by the use of a stainless steel laboratory knife. The seeds were removed, washed clean with distilled water and sundried for two weeks. The shells of the seeds were removed; de-shelled seeds were further broken down into smaller parts, sundried for two weeks, dried further in an oven at 700C and then pulverized by the use of a laboratory electric blender. About 250g of the crushed seeds were packed in a thimble and placed in a soxhlet extraction apparatus. 600ml of n-hexane was used to extract the oil from the pulverized seeds in the column. The extraction was left for 7 hours and stopped when complete. The obtained cake was washed with distilled water to remove residual n-hexane present, dried in an oven till a constant weight and the sieved through a 200µm mesh sieve to obtain Citropsis articulata seed powder (CASP). The CASP was used as the natural coagulant in this study and stored in a pre-treated air tight container.
Textile effluent collection and characterization
The textile effluent was collected from the effluent channel of a textile industry located in Ibadan, Oyo State, Nigeria. The samples were placed in pre-treated sample bottles and placed in an ice box to preserve the characteristics of the wastewater. The bottles were first rinsed with the sample of the effluent before collection. The physicochemical characteristics of the collected textile effluents were determined by standard methods [17], with the use of analytical grade chemicals obtained from sigma-Aldrich. Heavy metal concentration in the in the effluent was determined by the use of the Atomic Absorption Spectrophotometer (AAS) (Buck Scientific Model 210VGP). Turbidity of the effluent was measured by a turbidity meter (HACH Model-2100, USA) and was expressed in nephelometric turbidity units (NTU). The pH was determined by the use of a pH meter (DELTA 320 Model).
Algorithm of coagulation experiment
The coagulation-flocculation experiment was carried out using the standard jar test apparatus. The flow chart showing the coagulation-flocculation process of the bio-coagulant for textile effluent treatment is shown in Figure 1. Standard 500mL beakers were used to study the effect of coagulant dose, flocculation time and temperature on coagulation. To study the effect of coagulant dosage, the textile effluent was treated with different doses of CASP (2.0, 4.0, 6.0, 8.0, 10.0 g/L) at the natural effluent pH of 8.4, flocculation time 120 min and temperature 300K. This was performed by adding each dose of CASP to each of the beaker containing 250mL of the effluent and the jars were then placed in the jar test kit with the stirrers lowered into each. The stirring speed was set at 150rpm for rapid mixing for 2 min and 70rpm for slow mixing for 10 min followed by 120 min of settling. The supernatant sample was then withdrawn by a syringe from approximately 2cm below the liquid level for analysis for COD and turbidity. From the results obtained, the dosage of CASP with highest reduction of COD and turbidity was selected for subsequent experiment.
The effect of flocculation time was studied by treating the textile effluent with 10g/L dosage of CASP at effluent pH 8.4 and temperature 300K following the same procedure in the jar test experiment above. However, the settling time was varied (15, 30, 45, 60, 75. 90, 105, 120 min). To determine the influence of temperature on coagulation, the effluent was treated with 10g/L dosage of CASP, effluent pH 8.4, flocculation time 120 min but varying the temperature (300, 305, 310, 315, 320K) using a thermo-stated water bath. The jar test procedure outline above was followed. The natural pH of the effluent was maintained by the addition of 0.1 M NaOH or 0.1 M HCl when required. At the end of the coagulation-flocculation experiment, the supernatant were analysed for turbidity and COD. These parameters were determined before and after the experiment. All experiments were conducted in duplicate to ensure reproducibility of the results and the mean value calculated. The Turbidity or COD percentage reduction was calculated from the Eq. (1):
% Reduction = [(A0-A)/A0]100 (1)
Where: A0 - the initial turbidity (NTU) or COD (mg/L); A - the final values obtained.

Figure 1. Flow chat of the coagulation-flocculation process
Results and Discussion
Physicochemical characterization of textile effluent
The result of the characterization and heavy metal concentration of the textile effluent is presented in Table 1.
Table 1. Physicochemical characterization and heavy metal composition of the textile effluent
|
Parameter |
Value |
WHO Limits |
|
pH |
8.4 |
6.5-8.5 |
|
Temperature (0C) |
31.1 |
<40 |
|
Colour |
Brown |
Colourless |
|
Turbidity (NTU) |
254.7 |
5 Max |
|
EC (µS/cm) |
3174.3 |
1400 Max |
|
DO (mg/L) |
3.01 |
>5.0 |
|
BOD (mg/L) |
728.2 |
15-50 |
|
COD (mg/L) |
1402.7 |
40-250 |
|
TDS (mg/L) |
2824.1 |
500-1500 |
|
Calcium |
1503.6 |
75-200 |
|
Magnesium |
1641.9 |
30-150 |
|
TH (mg/L) |
1007.8 |
100 |
|
TA (mg/L) |
692.7 |
100-500 |
|
Chloride (mg/L) |
278.5 |
600 |
|
Sulphate (mg/L) |
678.4 |
200-600 |
|
Fe |
48.21 |
1.0 |
|
Zn |
6.48 |
5.0 |
|
Cu |
8.13 |
1.0 |
|
Mn |
4.28 |
0.1 |
|
Ni |
1.08 |
0.05 |
|
Cd |
0.74 |
0.005 |
|
Cr |
2.32 |
0.05 |
|
Pb |
0.34 |
0.05 |
It was clearly seen that all the parameters of the textile effluent except the pH, temperature and chloride exceeded the standard permissible limit recommended by the World Health Organization (WHO) [18]. The high value of contamination of the textile effluent is due to the use of a wide range of various chemicals such as dextrin, waxes, starch, gums, glucose, pectin, acetic acid, fatty acid, alcohol, soap, detergent, sodium hydroxide, chlorides, sulphites, sulphides, sulphates, carbonates, dyes, pigments, methyl cellulose, resins, fluorocarbons, silicones, peroxides, gelatines etc. in wet processes of the textile industry [2]. Similar result has been reported previously [19, 20].
pH is a simple but very important parameter as chemical reactions in most aquatic systems are affected by its changes. The pH of the textile effluent was however within the WHO permissible limit for wastewaters [18]. pH is also known to have an indirect effect on health as it affects the removal of viruses, bacteria and harmful microorganisms [21]. The alkaline nature of the textile effluent is due to the materials utilized in the textile effluent such as soap detergents and NaOH.
The temperature of an effluent is also very important as it controls the behavioural characteristics of organisms as well as the solubility of salts and gases in water [22]. Biochemical reactions of aquatic organisms depend on temperature and increase in temperature of water promotes chemical interactions due to insolubility of gases like oxygen, resulting in bas odor or taste [23]. It has been reported that textile and other dye wastewaters are produced at relatively high temperatures [20]. However, the temperature of the textile effluent was within the WHO standard recommended range [18, 20, 21].
Turbidity is an optical determination of the clarity of water. Turbid water will appear cloudy, murky or collared, affecting the physical look of the water. The brown colour observed for the textile effluent indicated clearly the turbidity of the effluent. Suspended solids and dissolved collared materials reduce the clarity of water by creating a muddy appearance. Turbidity measurements are often used as an indication of water quality based on the clarity and suspended solids in the water [24]. Turbidity results from the presence of suspended sediments such as clay, silt, inorganic matter, organic materials such as plankton, algae and decaying materials. In addition to suspended materials, turbidity also includes collared dissolved organic matter, fluorescent dissolved organic matter and dyes [25]. Therefore, since turbidity reveals to a great extent the pollution index of any water or wastewater it was chosen and analysed in this study to investigate the potential of CASP as coagulant for the effluent. The turbidity of the textile effluent obtained far exceeded the maximum recommended level by WHO [18, 26]. And fell in the range (>150NTU) reported for high turbid water [26]. This indicated that the textile effluent is highly polluted. The high Electrical Conductivity (EC) obtained is due to the low Dissolve Oxygen (DO) of the textile effluent [21].
DO is also one of the most important measure of water quality as hydrogen sulphide is formed in conditions of deficient oxygen in the presence of sulphate and organic matter [22]. The DO level of the textile effluent was below the recommended WHO limit indicating an unsuitable water which could be harmful to aquatic lives when discharged into receiving water bodies. The Biochemical Oxygen Demand (BOD) and Chemical Oxygen Demand (COD) inversely affects the DO, the low DO recorded was due to the very high BOD and COD values obtained. The BOD test provides information on the total amount of microorganisms present in the effluent and the nutrients available for them [21]. The BOD is an indication of organic load in the effluent as indicated by the high values of Total Dissolved Solid (TDS) obtained [27]. The COD is a measure of the capacity of water to consume oxygen during the decomposition of organic matter as well as the oxidation of inorganic materials such as ammonia and nitrate. COD does not measure the oxygen-consuming potential associated with certain dissolved organic compounds such as acetate unlike the BOD. The COD however reveals the pollution index of most industrial effluent than the BOD as the latter only measures the amount of oxygen consumed by microbial oxidation. In this regard the COD was also selected and analysed to evaluate the potential of CASP as natural coagulant for the textile effluent.
The main ions responsible for water hardness are the presence of calcium and magnesium and the occurrence of smaller quantities of manganese, iron, strontium and aluminium [28]. As observed from Table 1, the concentration of calcium, magnesium and iron (Fe) in the textile effluent was 1503.6, 1641.9 and 48.21 mg/L respectively and these values greatly exceeded the standard permissible limit prescribed by WHO [18, 21]. The high concentration of the ions resulted in the high value of Total Hardness (TH) of 1007.8 mg/L which exceeded the WHO permissible limit of 100 mg/L [22]. The high value of the TH recorded can lead to scale formation in some industrial plants and difficulty of soap to form lather during cleaning processes. The Total Alkalinity (TA) of 692.7 mg/L was slightly higher than the WHO permissible range of 100-500 mg/L [21] and must have resulted from the of some alkaline substances in textile operations. Chloride concentration of 278.5 mg/L was within the WHO maximum permissible limit while that of sulphate was higher than the recommended limit [18].
The effluent was also assessed for heavy metals concentration (Table 1). The heavy metals Fe, Zn, Cu, Mn, Ni, Cd and Pb were all above the WHO recommended standard permissible range [18, 21,22]. It has been reported that one of the major problems associated with textile effluent is the high concentration of metal ions arising from high amount of materials used in the dyeing process from metal containing dyes [20]. The heavy metals are toxic at certain concentrations to living organisms, persistent in nature, non-biodegradable and bio-accumulate in food chain resulting in various illnesses to living organisms and humans [29].
Effect of CASP dosage on coagulation
The dosage of coagulant represents one of the most important factors to be considered in order to determine optimum conditions for the use of a coagulant in coagulation-flocculation experiments. The use of overdose or insufficient amounts of the coagulants can result in inefficient performance of the coagulant in the process [30]. It is therefore significant to establish the optimum dosage so as to minimize the cost of dosing and the sludge formation in order to obtain optimum coagulating performance [2]. The influence of the dosage of coagulant (CASP) on the percentage reduction of turbidity and COD from the textile effluent is presented in Figure 2.
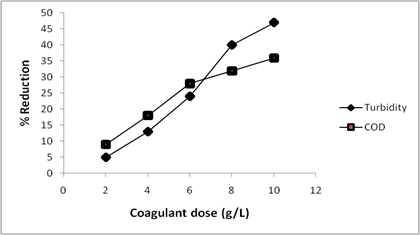
Figure 2. Effect of CASP dosage as natural coagulant for textile effluent
From the result, an increase in the percentage reduction of both turbidity and COD with increase in dosage of CASP from 2.0-10.0 g/L was recorded. COD had higher percentage reduction at lower doses of CASP but that of turbidity became higher at higher doses of 8.0-10.0 g/L. At the highest dosage of 10 g/L used in this study, percentage reduction of 47.3 and 36.4 were obtained for turbidity and COD respectively. The coagulation process is basically a surface phenomenon; therefore coagulation performance can be significantly affected by the surface charge due to the mass of the coagulant used [9]. Therefore the increase in the percentage reduction of turbidity and COD with increasing dosage of CASP is due to increase in surface charge for destabilization of the colloid suspension favouring flocculation process. The result of this study is however contrary to that of some researchers in which an initial increase in percentage reduction followed by a decrease with increase in coagulant dose was obtained [2, 9, 30]. The reason in their cases was attributed to the fact that calcium ions in solution can form complexes with certain inorganic groups on the coagulant and on the particle surfaces. As a result, the presence of excess coagulant made the suspended particles positively charged causing mutual repulsion. Therefore further increase in coagulant dose resulted in the re-dispersal of flocs and the rise in turbidity [9, 31]. This implies that the steady increase in percentage reduction in this experiment indicated that CASP was not in excess and hasn’t reached the optimum. This may be due to the very high turbidity of 254.7NTU and COD of 1402.7 mg/L when compared to those of other reports [9, 30, 26]. However, the dosage was not increase any further since it is expected that an efficient coagulant should have attained an optimum when such high doses of 10g/L is used.
Influence of flocculation time
The flocculation time which is the time required for formation and settling of the macro flocs is an important parameter given due consideration in an effluent treatment plant involving coagulation-flocculation processes. This is because the settling time of the flocs affects the overall cost as well as the efficiency of the process and is useful in design of a coagulation-flocculation treatment plant. The result for the effect of flocculation time on the percentage removal of turbidity and COD using CASP as coagulant is shown in Figure 3.
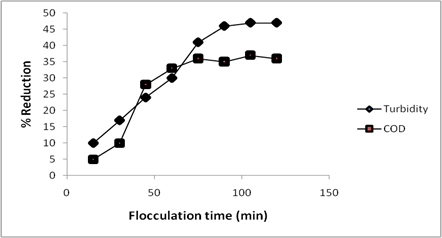
Figure 3. Effect of flocculation time on the use of CASP as natural coagulant for textile effluent
It was observed in Figure 3 that a steady increase in the percentage reduction of turbidity and COD with increase in flocculation time was recorded after which it became constant at equilibrium. The COD had a slow rate of reduction at the initial time but increased rapidly in the latter stages attaining equilibrium at 75 min. However, turbidity reduction attained equilibrium at 90 min of the flocculation experiment. The initial increase was attributed to the coming together, formation and settling of the flocs which progressed with time attaining equilibrium beyond which no significant formation or settling of flocs was achieved. At equilibrium it is believed that maximum flocs formation and settling has been achieved. A flocculation time of 120 min was selected and utilized in this study to ensure equilibrium flocculation time was achieved for all the experiments for COD and turbidity reduction. Similar results have been reported elsewhere [32, 33].
Effect of temperature on coagulation
Temperature is also an important factor that affects the coagulation, flocs formation as well as settling coagulation-flocculation experiments. In this regard, the effect of temperature on the coagulation-flocculation performance of CASP was investigated in this study.
Figure 4 showed the influence of temperature on the percentage reduction of turbidity and COD from textile effluent using CASP as natural coagulant.
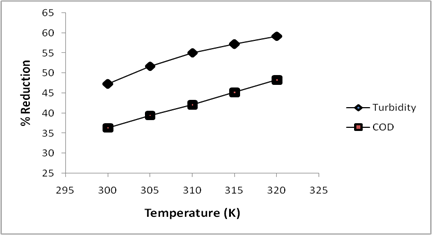
Figure 4. Effect of temperature on the use of CASP as natural coagulant for textile effluent
An increase in the removal of both turbidity and COD with increase in temperature from 300 to 320K was obtained. This indicated that the coagulation process is endothermic in nature and high temperatures favour the process. However, due to the increasing in cost of maintain higher temperatures in coagulation-flocculation treatment process, the room temperature of 300K was maintained in the experiments in this study. Similar coagulation-flocculation temperature effect has been reported in the destabilization of cutting oil emulsions using inorganic salts as coagulants [34].
Conclusion
The physicochemical and heavy metal analysis of the textile effluent showed a highly polluted wastewater in which the parameters exceeded the WHO recommended permissible limits, except the pH, temperature and chlorides which were within the limits. An increase in the percentage reduction of turbidity and chemical oxygen demand with increase in coagulant dose, flocculation time and temperature was recorded. Importantly, the Citropsisarticulata seed powders used as natural coagulant recorded highest percentage reduction of turbidity and COD of 59.2% and 48.3% respectively at coagulant dosage of 10mg/L and temperature of 320K. However, at room temperature of 300K the optimum reduction of turbidity and COD was 47.3 and 36.4% respectively. Although the turbidity and COD of the textile effluent was very high, the use of such high dose (10g/L) of the coagulant did not establish a percentage reduction of 60% for both turbidity and COD. This suggested that Citropsisarticulata could only be utilized in the reduction of turbidity and COD in high turbid waters but not an alternative to highly efficient commercial coagulants like alum.
References
1. Muthukumar K., Sundaram P.S, Anantharaman M., Basha C.A., Treatment of textile dye wastewater by using an electrochemical biopolar disc stack reactor, J. Chem. Technol. Biotechnol., 2004, 79, p. 1135-1141.
2. Patel H., Vashi R.T., Comparison of naturally prepared coagulants for removal of COD and color from textile wastewater, Global NEST Journal, 2013, 15, p. 522-528.
3. Yaser A.Z., Cassey T.L., Hairul M.A., Shazwan A.S., Current review on the coagulation/flocculation of lignin containing wastewater, Int.. J. Wat. Res., 2014, 4, p. 1-6.
4. Antov M.G., Sciban M.B., Prodanovic J.M., Evaluation of the efficiency of natural coagulant obtained by ultrafiltration of common bean seed extract in water turbidity removal, Ecol. Eng., 2012, 49, p. 8-52.
5. Zahrim A.Y., Tizaoui C., Hilal N., Evaluation of several commercial synthetic polymers as flocculant aids for removal of highly concentrated C.I. acid black 210 dye, J. Hazard Mater., 2010, 182, p. 624-630.
6. Bratskaya S., Schwartz S., Chervonetsky T., Comparative study of humic acids flocculation with chitosan hydrochloride and chitosan glutamate, Water Res., 2004, 38, p. 2955-2961.
7. Devrimci H.A., Yuksel A.M., Sanin F.D., Algal alginate: a potential coagulant for drinking water treatment, Desalination, 2012, 299, p. 16-21.
8. Anastasaki K., Kalderis D., Diamadopoulos E., Flocculation behaviour of mallow and okra mucilage in treating wastewater, Desalination, 2009, 249, p. 786-791.
9. Ramavandi B., Treatment of water turbidity and bacteria by using a coagulant extracted from Plantago ovata, Water Resources and Industry, 2014, 6, p. 36-50.
10. Antov M.G., Sciban M.B., Petrovic N.J., Proteins from common bean (Phaseolus vulgaris) seed as natural coagulant for potential application in water treatment removal, Bioresource Technol., 2010, 101, p. 2167-2172.
11. Chethana M., Gayatri S.L., Bhandari V.M., Ranade V.V., Raja S., Application of biocoagulant acanthocereus tetragonus (triangle cactus) in dye wastewater treatment, J. Environ. Res. Develop., 2015, 9, p. 813-821.
12. Bhuptawat H., Folkard G.K., Chaudhari S., Innovative physicochemical treatment of wastewater incorporating Moringa Oleifera seed coagulant, J.Haz.Mat, 2007, 142, p. 477-82.
13. Oladoja N.A., Aliu Y.D., Evaluation of plantain peelings ash extract as coagulant aid in the coagulation of colloidal particles in low pH aqua system, Water Qual. Res. J. Can., 2008, 43, p. 231-238.
14. Diaz A., Rincon N., Escovihuela A., Fernandez N., Chacin E., Foister C.F., A preliminary evaluation of turbidity removal by natural coagulants indigenous to Venezuela, Process Biochemistry, 1999, 35, p. 391-395.
15. Sciban M., Klasnja M., Antov M., Skrbic B., Removal of water turbidity by natural coagulants obtained from chestnut and acorn, Biores. Tech., 2009, 100, p. 6639-6643.
16. Sanghi R., Bhattacharya B., Singh V., Cassia angustifolia seed gum as an effective natural coagulant for de-colorization of dye solution, Green Chemistry, 2002, 4, p. 252-254.
17. Association of Official Analytical Chemist (AOAC), In: Official Methods of Analysis, 20th ed., Rockville, MD, 2005.
18. World Health Organization (WHO), World Report: Shaping the Future, Geneva, 2003.
19. Kuai L., De Vreese I, Vandevivere P., Verstraete W., GAC-Amended UASB reactor for the stable treatment of toxic textile wastewater, Environ. Technol., 1998, 19, p. 1111-1117.
20. Joshi V.J, Santani D.D., Physicochemical characterization and heavy metal concentration in effluent for textile industry, Uni. J. Environ. Res. Technol., 2012, 2, p. 93-96.
21. Ani J.N, Nnaji N.J., Okoye C.O.B., Onkwuli O.D., The coagulation performance of okra mucilage in an industrial effluent by turbidity, Inter. J. Chem. Sci., 2012, 10, p.1293-1308.
22. Akpomie K.G., Dawodu F.A., Physicochemical analysis of automobile effluent before and after treatment with an alkaline-activated montmorillonite, J. Taibah. Uni. Sci., 2015, 9, p. 465-476.
23. Akan J.C., Abdulrahman F.L., Dimari G.A., Ogugbuaja V.O., Physicochemical determination of pollutants in wastewater and the vegetable samples along the Jankara wastewater channel in Kano metropolis, Kano State, Nigeria, Eur. J. Sci. Res., 2008, 23, p. 122-133.
24. Gray J.R., Gylsson G.D., Turcios L.M., Schwarz G.E., Comparability of suspended sediment concentration and total suspended solids data, USGS Water-Resources Investigations Report 00-4191, Reston, Geological Survey, 2000.
25. Fink J.C., Establishing the relationship between sediment concentration and turbidity: In the effect of urbanization on Baird Creek, Green Bay, WI (Thesis), 2005.
26. Muhammad I.M., Abdulsalam S., Abdulkarim A., Bello A.A., Water melon seed as a potential coagulant for water treatment, Glob.J.Res.Eng.C.Chem. Eng., 2015, 15, p. 17-23.
27. Mishra A., Pal S., Polyacylonitrile-grafted okra mucilage: a renewable reservoir to polymeric materials, Carbohydrate Polymers, 2007, 68, p. 95-100.
28. Agarwal M., Srinivasan R., Mishra A., Study on flocculation efficiency of okra gum in sewage wastewater, Macromol. Mater. Eng., 2001, 286, p. 560-563.
29. Dawodu F.A., Akpomie K.G., Simultaneous adsorption of Ni (II) and Mn (II) ions from aqueous solution unto a Nigerian kaolinite clay, J. Mater. Res. Tech., 2014, 3, p. 129-141.
30. Nourmoradi H., Rahmati Z., Javaheri M., Moradnejadi K., Noorimotlagh Z., Effect of praestol as a coagulant aid to improve coagulation-flocculation in dye containing wastewater, Global NEST Journal, 2015, 18, p. 38-46.
31. Ramavandi B., Farjadfard S., Removal of chemical oxygen demand from textile wastewater using natural coagulant, Korean J. Chem. Eng., 2014, 31, p. 81-87.
32. Fu Y., Yu S.L., Characterization and coagulation performance of solid poly-silicic-ferric (PSF) coagulant, J. Non-Crystal Solids, 2007, 353, p. 2206-2213.
33. Omar F.M., Rahman N.N.A., Ahmadi A., COD reduction in semiconductor wastewater by natural and commercialized coagulants using response surface methodology, Water Air Soil Pollution, 2008, 195, p. 345-352.
34. Rios G., Pazos C., Coca J., Destabilization of cutting oil emulsions using inorganic salts as coagulant, Colliod Surface A., 1998, 138, p. 383-389.