Engineering, Environment
Synthetic process of vat-dyes of tetraaza derivatives of three-branched phenothiazine heterocycles
Benjamin EZEMA 1*, Chidimma EZEMA 2, Kovo AKPOMIE 1*, David UGWU 1
1 Department of Pure and Industrial Chemistry, Faculty of Physical Sciences, University of Nigeria, Nsukka, 410002, Enugu State, Nigeria
2 Centre for Energy Research and Development, University of Nigeria, Nsukka, 410002, Enugu State, Nigeria
E-mail(s): benjamin.ezema@unn.edu.ng; kovo.akpomie@unn.edu.ng
* Corresponding Author phone: +2348066072738, +2348037617494
Received: July 10, 2018 / Accepted: December 03, 2018 / Published: December 30, 2018
Abstract
Tetra-aza derivatives of three-branched phenothiazine compounds were synthesized. The key intermediate 6-chlorobenzo[a]-9,ll-diazaphenothiazin-5-one (16) was prepared by condensation reaction of 4-aminoprimidin-5-thiol (14) with 2,3-dichloro-l,4-naphthoquinone (15) in alkaline medium. The 6,8,13,15-tetraazabenzo[a][1,4] benzothiazino [3, 2-c] phenothiazine (20) and the derivatives (23-25) were synthesized by base catalyzed condensation reactions of the key intermediate with substituted pyrimidin-5-thiols (11,14,21,22). The compounds were characterized by elemental and spectral analysis.
Keywords
4-aminopyrimidin-5-thiol; 4-amino-6-hydroxyprimidin-5-thiol; 2-amino-5- bromopyrazin-3-thiol, 4, 6-diaminopyrimidin-5-thiol, 2,3-dichloro-1,4-naphthoquinone
Introduction
Phenothiazine also called dibenzothiazine or thio-diphenylamine is a yellow crystalline compound soluble in hot acetic acid, benzene and ether. It is a three ring structure compound in which two benzene rings are joined by sulphur and nitrogen atom at nonadjacent positions. It is obtained by fusing diphenylamine with sulphur. The chemistry of phenothiazine and its derivatives have been extensively studied because of their versatile utility in industry, agriculture and medicine [1]. In industries, it has been applied as dyes and pigments antioxidants in lubricants and fuels and insecticide in agriculture [2,3]. Phenothiazine is also a bioactive heterocyclic compound of Pharmaceutical importance and possesses different biological activities ranging from antibacterial, antifungal, antitubercular, anti-inflammatory, antimalarial, antipsychotic, antitumor, and antihistaminic. They are also used as semi conducting materials in electronics [4-11].
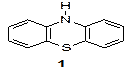
Figure 1. Phenotiazine
Extensive structural modification of Figure 1 and its derivatives is still in progress in an attempt to obtain new derivatives having more desirable properties. The derivatives in which benzene or six member heterocyclic ring(s) is/are fused at different positions of the carbon chains of ring A and C has given rise to the reports of some of these derivatives benzo[a] phenothiazine in Figure 2 (2), benzo[c] phenothiazine in Figure 2 (3), dibenzo[a,h] phenothiazine in Figure 2 (4), and dibenzo[c,h] phenothiazine in Figure 2 (5) [11].

Figure 2. Phenotiazine derivatives: benzo[a] phenothiazine (2), benzo[c] phenothiazine (3), dibenzo[a,h] phenothiazine (4) and dibenzo[c,h] phenothiazine
Also known are triangular or “three branched” of the types 14H-dibenzo [a,c] phenothiazine in Figure 3 (6) and benzo[a] [1,4] benzothiazino3, [2-c] phenothiazine in Figure 3 (7). Okafor and co-workers ventured into the synthesis of the first set of triazaphenothiazine systems [2].
The aim of this research was to synthesize vat dyes of tetraaza derivatives of three branched phenothiazine heterocycles by a suitable process. The authors hereby describe the synthesis of tetraaza derivatives of three-branched phenothiazine heterocycle of the Figure 3 (7).
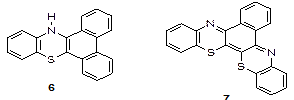
Figure 3. Tetraaza derivatives of three-branched phenothiazine heterocycle
Materials and Methods
Algorithm of experiment
The reagents were of analytical grades from Sigma-Aldrich. They were used without further purification. Melting points were recorded on electro-thermal apparatus in open capillaries. The reactions were monitored with TLC and also the purity was ascertained with thin layer chromatography. Infrared spectra analysis was obtained on SHIMADZU FT-IR 8400S spectrophotometer using KBr discs in the region of 4500-500 cm-1. The 1H NMR and 13C NMR were recorded on Brucker instrument 400 MHz; chemical shifts were reported on ppm scale. Microanalysis was obtained on Heraous CHN rapid analyzer. The general flow chat of the synthetic process is shown in Figure 4.
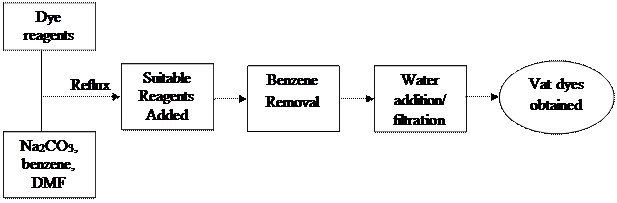
Figure 4. Flow chat of the synthetic process of vat dyes of tetraaza derivatives
4-Amino-5-bromo-6-hydroxypyrimidine (9)
4-Amino-6-hydroxypyrimidine in Figure 5 (8) (15.00 g. 0.135 mol) was dissolved in methanol (50%, 100 mL) contained in a 500 mL flask and sodium bicarbonate (30.00 g) was added. Bromine (6.0 mL) was added drop wise to the reaction mixture from a dropping funnel with continuous stirring within 40 min.
Additional small amount of sodium bicarbonate (8.00 g) was added 20 min later and the reaction mixture allowed for 2 h with constant stirring at room temperature.
At the end of the reaction, the semi-solid product was allowed to stand overnight. Water was added to dissolve the inorganic material and filtered. The residue was transferred into a 250 ml beaker containing warm water and boiled with stirring till complete dissolution, little quantity of activated carbon was added and the solution boiled for further 10 min. It was filtered hot and the filtrate allowed cooling to give a cream white crystals mp 267 0C. IR (KBr) νstretch: 3445, 3320, 3040, 1570, 1480, 1220, 1150, 1100, 860, 760.
4-Amino-6-hydroxy-5-thiocyanatopyrimidine (10)
Into a reaction flask containing boiled water (300 mL) and connected to a reflux condenser was added 4-amino-5-bromo-6-hydroxypymidine in Figure 5 (9) (10.0 g, 0.59 mol). The mixture was boiled till complete dissolution. Potassium thiocyanate (12.00 g) in water (25 mL) was added and the solution refluxed for 2 h. It was allowed to cool and then filtered. The residue was boiled in acetone, treated with activated charcoal and filtered after cooling to give bright orange solid product mp 3090C. IR (KBr, cm-1) νstretch: 3455, 3320, 3050, 16201570, 1450, 12501130, 1010, 750,710
4-Amino-6-hydroxypyrimidine-5-thiol (11)
Into a 250 mL reaction flask attached to a reflux condenser was added 4-amino-6-hydroxy-5-thiocyanatopyrimidine in Figure 5 (10) (5.0 g, 0.55 mol). Aqueous solution of sodium hydroxide (20 %) was added and the mixture refluxed in a sand bath for 24 h.
At the completion of the reflux period, activated carbon was added to the mixture and boiled for additional 20 min. It was filtered hot and allowed cooling; later neutralized with acetic acid in an ice bath by maintaining the temperature below 100C.
An orange precipitate was formed and allowed to settle; it was filtered and recrystallized from acetone and dried in a desiccator, mp 3180C. IR (KBr, cm-1) νstretch: 3435, 3320, 3030, 1562, 1460, 1230, 1120, 791, 760 and 651.
4-Amino-5-thiocyanatopyrimidine (13)
4-Aminopyrimidine in Figure 5 (12) (10.0 g, 0.11 moll) was added into a one litter flask attached to a reflux condenser, mechanical stirrer and dropping funnel. Glacial acetic acid cooled to 180C was added and the mixture immersed in ice-salt bath. Sodium thiocyanate (15.0 g) was added with stirring while maintaining the temperature at -5 0C and 00C.
Bromine (5.0 mL) was introduced in droplets to the mixture for over 1 hour and stirring was continued for a period of 5 h while maintaining the temperature at near 00C was allowed to stand overnight, water was added and mixture warmed to 800C. It was filtered and the filtrate preserved; the orange solid was extracted with acetic acid.
The combined acetic acid extracts with the preserved filtrate was neutralized with concentrated ammonia while cooling and maintaining the temperature below 300C.
The crude product was then filtered and recrystallized from methanol to give 4-amino-thiocyanatopyrimidine as yellow solid m.p. 201-2020C. IR (KBr, cm-1) νstretch: 333S, 3220, 3040, 1572, 1465, 1240, 1120, 791 and 760.
4-Aminopyrimidine-5-thiol (14)
4-Amino-5-thiocyanatopyrimidine in Figure 5 (10) (8.0.g, 0.52 mol) was added into a reaction flask containing 40 g of potassium hydroxide in 100 mL of water. The mixture was warmed for dissolution and refluxed for 13 h, little amount of carbon was added and refluxed further for I5 min. It was filtered hot and allowed to cool. It was neutralized with glacial acetic acid in ice-salt bath by maintaining the temperature below 100C. It was filtered and the residue purified from methanol to give yellow crystals, mp 3300C. IR (KBr, cm-1) νstretch: 3450, 3313, 3040, I590 1470, 1300, 1120, 1050, 770 and 728.
6-Chlorobenzo[a]-9, 11-diazaphenothiazine-5-one (16)
4-Aminopyrimidine-5-thiol in Figure 5 (14) (4.0 g, 0.31 mol) was added in a 250 mL reaction flask containing anhydrous sodium carbonate (3.34 g, 0.31 moles), benzene (120 mL) and DMF (40 mL) and the mixture boiled for 1 h.
2,3-Dichloro-I,4-naphthoquinone (15) (7.14 g, 0.31 moles) was added and the mixture was refluxed with continuous stirring for 6 h at 800C. Benzene was distilled off and water was added to dissolve the inorganic materials; chilled overnight and filtered.
The solid residue was boiled in methanol/acetone and treated with activated carbon to give in Figure 5 (16) as red powder mp > 3100C. IR (KBr, cm-1) νstretch: 3060, 1661, 1594, 1460, 1378, 1211, 1101, 1023,728 and 650. 1H NMR (400MHz/DMSO): d 9.l0 (s, 1H), 8.80 (s, 1H) 7.90-7.87 for (m, 4H). l3C-NMR (400MHz/CDCl3): d 193.7 (C=O), 150.4, 148.6 136.l, 131.9, 128.6, 127.2, 126.4, 125.3, 123.6,121.7,118.3,117.2,115.5.
Analysis: Calculated for C14H6N3ClOS: (%) C, 56.10; H. 2.02; N, 16.94; Cl, 11.83, S, 10.70; (Found: C, 56.07; H, 2.05; N, 16.98; Cl, 11.83; S, 10.67).
General procedure for the synthesis of branched complex compounds: 6, 8, 13, 15-Tetraazabenzo[a] [1, 4] benzothiazino [3,2-c] phellothiazine (22)
4-Aminopyrimidine-5-thiol in Figure 5 (14) (1.50 g, 0.12 mol) was poured into a 250 mL reaction flask attached to reflux condenser. Anhydrous sodium carbonate (1.25 g, 0.12 mol), benzene (120 mL) and dimethylformamide (30 mL) were added and the mixture was warmed for 1h. Then 6-chlorobenzo [a]-9, 11-diazaphenothiazin-5-one in Figure 5 (16) (2.70 g, 0.12 mol) was added and the mixture refluxed in water bath for 10 h with stirring. During the reaction period, it was spotted on coated thin layer chromatography plate every 1 h interval. Benzene was removed by vacuum distillation and warm water was added and mixture transferred into a beaker, stirred and allowed to cool. It was filtered and residue re-dissolved in boiling methanol/acetone.
Little amount of activated carbon was added and boiled for additional 5 min, filtered and allowed cooling to give 6,8,13,15-tetraaza heterocyclic product in Figure 5 (20) as purple red powder; mp 305 0C; IR (KBr, cm-1) νstretch: 3030, 1600,1558,1450,1288,1161,1011,782 and 710. 1H NMR (400MHz/DMSO): d 9.15 (H-7, H-14, s), 8.90 (H-9, H-12, s) and 7.90-7.86 (4R m). 13C NMR (400MHz/CDCl3), d: 158.8, 157.4, 155.3, 154.7, 149.3, 139.43, 134.6, 132.1. 117.4. 126.8. 124.7, 120.9, 120.1, 118.7, 118.5 and 117.5. Analysis: Calculated for C18H8N6OS2: C, 58.05. H. 2.17. N. 22.57, S, 17.22, Found: C, 58.08, H, 2.15, N, 22.60, S, 17.20.
9-Hydroxy-6, 9, 13, l5-stetraazabenzo [a] [1, 4] benzothiazino [3,2-c] phenothiazine (25)
Dark red powder; m.p 3200C. 1H NMR (400MHz/DMSO): d 9.50 (br, OH), 9.10 (H-14, s), 9.05 (H-7, s), 8.85 (H-11, s), 7.90-7.87 (4H, m). 13C NMR (400MHz/CDCl3): d 5158.1, 157.4, 155.3, 154.7, 149.3, 147.4, 144.6, 142.1, 137.4, 132.8, 131.7, 130.9, 130.1, 128.7, 123.5, 119.5. Analysis: Calculated for C18H9N8OS2: C, 55.66, H, 2.08, N, 21.64, S, 16.51. Found: C, 57.70, H, 2.05, N, 21.67, S, 16.48.
8-Bromo-6, 9, 13, 15-tetraazabenzo [a] [1, 4] bellzothiazino [3,2-c] phenothiazine (26)
Compound 26 crystallized out as a purple-brown crystals mp 2940C (dec.). 1H NMR (400MHz/DMSO): d 9.10 (H-14, s), 8.50 (H-7, s), 8.85 (H-11, s), 7.90-7.87 (4H, m). l3C NMR (400MHz/DMSO): d 168.1, 167.4, 165.3, 164.7, 159.34, 149.4, 144.6, 142.1, 137.4,132.8,131.7,130.9,130.1,128.7,123.5,122.5. Analysis: Calculated for C18H7BrN6S2: C, 49.90; H, 1.56; Br, 17.70; N, 18.62; S, 14.21; Found: C, 49.96; H, 1.54; Br, 17.74; N, 18.62; S, 14.24.
9-Amino-6, 8, 13, l5-tetraazabenzo[a] [1, 4] benzothiazino [3, 2-c] phenothiazine (27)
The colour of the compound was dark red-brown, mp 3050C. 1H NMR (400MHz/DMSO): d 9.10 (H-14, s), 8.10 (H-7, s), 8.85 (H-11, s), 7.90-7.87 (4H, m), 6.70 (NH2). 13C NMR (400MHz/DMSO): d 168.1, 167.40, 165.3, 164.7, 159.3, 149.4, 144.6, 142.1, 137.4, 132.8, 131.7, 130.9, 130.1, 128.7, 123. 5,122.5. Analysis: Calculated for C18H9N7S2: C, 55.80, H, 2.24, N, 25.31, S, 16.55. Found: C, 55.86, H, 2.19, N, 25.37, S, 16.58.
Results
and Discussion
4-Amino-6-hydroxypyrimidine in Figure 5 (8) was converted to 4-amino-5-bromo-6-hydroxy pyrimidine in Figure 5 (9) by treating with a solution of bromine in methanol and solid sodium bicarbonate. Further treatment of 9 with solution of potassium thiocyanate gave 4-amino-6-hydroxy-5-thiocyanatopyrimidine in Figure 5 (10). When compound in Figure 5 (10) was refluxed in 20 % aqueous sodium hydroxide and later neutralized with ethanoic acid, 4-amino-6-hydroxyprimidine-5-thiol in Figure 5 (11) was obtained.
On the other hand, 4-aminopyrimidine in Figure 5 (12) was treated with potassium thiocyanate in bromine and acetic acid solution to give 4-amino-5-thiocyanatopyrimidine in Figure 5 (13). Refluxing of compound 13 in 20 % aqueous solution of sodium hydroxide for 13 hours and then neutralization with acetic acid gave 4-aminopyrimidine-5-thiol in Figure 5 (14) as shown:
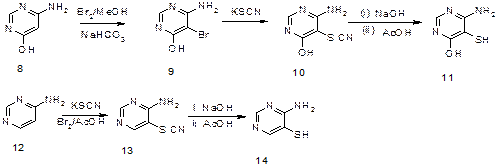
Figure 5. Synthesis of 4-aminopyrimidine-5-thiol
When 4-aminopyrimidin-5-thiol in Figure 6 (14) was treated with 2,3-dichloro-l,4-naphthoquinone in Figure 6 (15) in a mixture of benzene/DMF and anhydrous sodium carbonate as a catalyst and refluxed for 6 h, 6-chlorobenzo[a]-9,11-diazaphenothiazin-5-one in Figure 6 (16) was obtained. Result of elemental analysis in Figure 6 (16) agrees with the formula C14H6N3ClOS. Infrared spectrum gave strong bands at cm-1: 1665 (νC=O of cyclic ketone) and 1594, 1478 (νC=N, νC=C of aromatics). Compound in Figure 6 (16) exhibited the following 1H NMR chemical shift signals in ppm: 9.10 assigned to (H-10, s), 8.80 to (H-8, s) 7.90-7.87 to (4H, m). 13C NMR gave a prominent chemical shift value at ppm 193.7 assigned to C=O (in Figure 6).
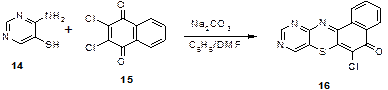
Figure 6. Synthesis of 6-chlorobenzo[a]-9,11-diazaphenothiazin-5-one
In Figure 7 compound 16 is probably formed by the devised mechanism (Figure 7):
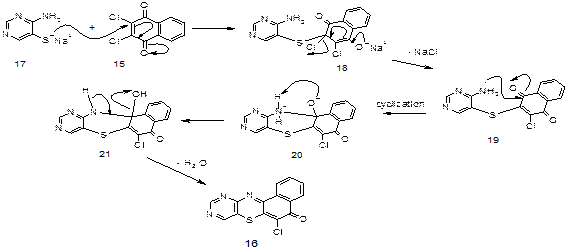
Figure 7. Devised mechanism for formation of 6-chlorobenzo[a]-9,11-diazaphenothiazin-5-one
When 16 (from Figure 8) was treated with a second molecule of 14 (from Figure 8) under the same base catalysed reaction condition, a tetraaza three-branched heterocycle characterized as 6,8,13,15-tetraazabenzo [a] [1,4] benzothiazino [3,2-c] phenothiazine (from Figure 8 (22)) was obtained. Result of elemental analysis of 22 (from Figure 8) is in agreement with the molecular formula C18H8N6S2. Infrared spectrum gave bands at 1590, 1540 cm-1 assigned to (aromatic νC=N, νC=C). Compound 22 (from Figure 8) exhibited proton chemical shift values in ppm as follows; 9.15 assigned to (H-7, H-14, s), 8.90 (H-9, H-12, s) and 7.90-7.86 (4H, m) respectively (Figure 8).
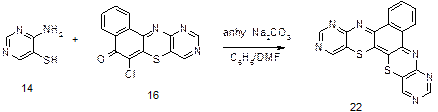
Figure 8. Synthesis of 6,8,13,15-tetraazabenzo [a] [1,4] benzothiazino [3,2-c] phenothiazine
Again when 16 was treated with 4-amino-6-hydroxyprimidin-5-thiol (11), 2-amino-5- bromopyrazin-3-thiol (23) and 4,6-diaminopyrimidin-5-thiol respectively (24); 9-hydroxy-6,9,13,15-tetraazabenzo [a][1,4] benzothiazino [3,2-c] phenothiazine (25), 8-bromo-6,9,13,15-tetraazabenzo[a][1,4] benzothiazino [3,2-c] phenothiazine (26) and 9-amino-6,8,13,15-tetraazabenzo [a][1,4] benzothiazino [3,2-c] phenothiazine (27) were obtained in good yields. Results of elemental analysis of the compounds agree with the molecular formulas: 25; C18H9N6OS2. 26; C18H7BrN6S2: 27; C18H9N7S2.
1H NMR spectrum of 25 showed chemical shift values in ppm at: 9.50 assigned to (OH, s), 9.10 (H-14, s), 9.05 (H-7, s), 8.85 (H-11, s), 7.90-7.87 (4H, m), 26; d 9.10 (H-14, s), 8.50 (H-7, s), 8.85 (H-11, s), 7.90-7.87 (4H, m) and 27; d 9.10 (H-14, s), 8.10 (H-7, s), 8.85 (H-11, s), 7.90-7.87 (4H, m), 6.70 (NH2). Their 13C-NMR values are presented in the experimental section (Figure 9);
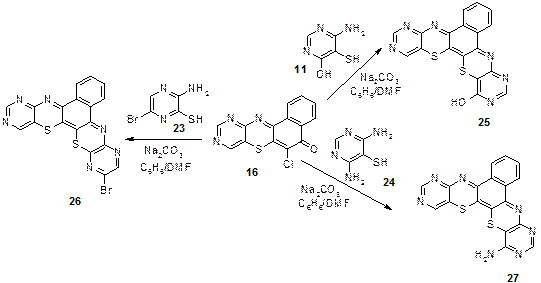
Figure 9. Synthesis of compounds 25, 26 and 27
The general proposed mechanism for the formations of the products is shown (Scheme 6): a nucleophilic attack by 17 on 16 to form an ionic intermediate 28 which eliminated (Cl-) to generate 29. Then 29 on intramolecular nucleophilic attack by amino group on the carbonyl and the cyclisation generated 30 which later lost a molecule of water to give 22 (Figure 10):
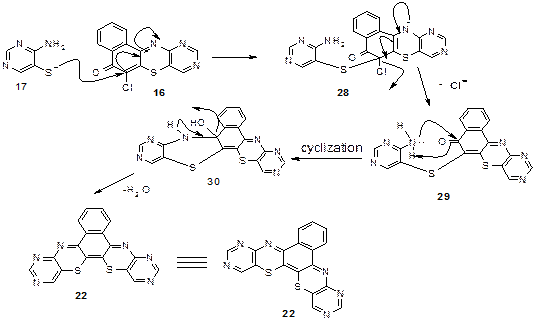
Figure 10. Mechanism for synthesis of compound 22
Conclusion
We have successfully synthesized tetraaza-benzothiazinophenothiazine compounds by base catalyzed condensation reactions. The compounds were characterized using infrared, proton and carbon-13 NMR spectroscopy and elemental analysis.
The structures are in agreement with the spectra. The compounds can be of industrial important as colourants and also as pharmaceuticals.
References
1. Adekola E.O., Ezema B.E., Ayogu J.I., Ugwu D.I., Ezema C.G., Abuekwu P.N., Ike C.O., Synthesis of benzothiazinophenothiazine derivatives and their antimicrobial Screening, Orient. J. Chem, 2014, 30 (4), p. 1493-1500.
2. Okafor C.O., Okerelu I.O., Okeke S.I., Vat dyes from three new heterocyclic ring, Dyes and Pigments, 1986, 8, p. 11-24.
3. Mitchell S.C., Mammalian metabolism of administered phenothiazine, Drug Meta. Rev., 1982, 13, p. 319-343.
4. Srivastava S.K., Dua R., Srivastava S.D., Synthesis and antimicrobial activity of [N1-(N- substitutedarylidene-hydrazino)-acetyl]-2-methyl-imidazoles and [N1-(4-substituted aryl-3-chloro-2-oxo-1-azetidinyl-amino)-acetyl]-2-methyl-imidazoles, Proc. Nat. Acad. Sci. India, Sec A, Phys. Sci., 2010, 80, p. 117-121.
5. Trivedi P.B., Undavia N.K., Dave A.M., Bhatt K.N., Desai N.C., Synthesis and antimicrobial activity of 4-oxothiazolidines, 4-oxoazetidines, malonanilic acid hydrazines and pyrazoline derivatives of phenothiazine, Indian J Chem., 1993, 32B (7), p. 760-765.
6. Viveros M., Amaral L., Design, synthesis, characterisation and anti-tubercular activity of some 2-Heterocycle-substituted phenothiazines, Int. J. Antimicrob Ag, 2001, 17, p. 225-234.
7. Kalkanidis M., Klonis N., Tilley L., Deady L.W., Novel phenothiazine antimalarial: Synthesis, antimalarial activities and inhibition of the formation of beta-haematin, Biochem. Pharmacol., 2002, 63 (5), p. 833-841.
8. Lin G., Medha K.K., Synthesis of the piperidinone metabolites of piperidine-type phenothiazine antipsychotic drugs via ruthenium tetraoxide oxidation, J. Heterocycl. Chem., 1991, 28, p. 215-223.
9. Motohasho N., Kawase M., Saito S., Sakagami H., Antitumor potential and possible targets of phenothiazine-related compounds, Curr. Drug Targets, 2000, 1, p. 237-245.
10. Kubota K., Kurebayashi H., Miyachi H., Tobe M., Onishi M., Isobe Y., Synthesis and structure-activity relationships of phenothiazine carboxylic acids having pyrimidine-dione as novel histamine H(1) antagonists, Bioorg. Med. Chem. Lett., 2009, 19 (10), p. 2766-2771.
11. Nowakowska-Oleksy A., Sołoducho J., Cabaj J., Phenoxazine based units- synthesis, photophysics and electrochemistry, J. Fluoresc., 2011, 21, p. 169-178.